Vectors and SI Units.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M Motion Intervention Booklet.Pdf (810.7KB)
Motion Intervention Booklet 1 Random uncertainties have no pattern. They can cause a reading to be higher or lower than the “true value”. Systematic uncertainties have a pattern. They cause readings to be consistently too high or consistently too low. 2 The uncertainty in repeat readings is found from “half the range” and is given to 1 significant figure. Time / s Trial 1 Trial 2 Trial 3 Mean 1.23 1.20 1.28 Mean time = 1.23 + 1.20 + 1.28 = 1.23666666 => 1.24 (original data is to 2dp, so 3 the mean is to 2 dp as well) Range = 1.20 1.28. Uncertainty is ½ × (1.28 – 1.20) = ½ × 0.08 = ± 0.04 s 3 The speed at which a person can walk, run or cycle depends on many factors including: age, terrain, fitness and distance travelled. The speed of a moving object usually changes while it’s moving. Typical values for speeds may be taken as: walking: 1.5 m/s running: 3 m/s cycling: 6 m/s sound in air: 330 m/s 4 Scalar quantities have magnitude only. Speed is a scaler, eg speed = 30 m/s. Vector quantities have magnitude and direction. Velocity is a vector, eg velocity = 30 m/s north. Distance is how far something moves, it doesn’t involve direction. The displacement at a point is how far something is from the start point, in a straight line, including the direction. It doesn’t make any difference how far the object has moved in order to get to that point. -

CAR-ANS PART 05 Issue No. 2 Units of Measurement to Be Used In
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila INTENTIONALLY LEFT BLANK CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

SI Base Units
463 Appendix I SI base units 1 THE SEVEN BASE UNITS IN THE INTERNatioNAL SYSTEM OF UNITS (SI) Quantity Name of Symbol base SI Unit Length metre m Mass kilogram kg Time second s Electric current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd 2 SOME DERIVED SI UNITS WITH THEIR SYMBOL/DerivatioN Quantity Common Unit Symbol Derivation symbol Term Term Length a, b, c metre m SI base unit Area A square metre m² Volume V cubic metre m³ Mass m kilogram kg SI base unit Density r (rho) kilogram per cubic metre kg/m³ Force F newton N 1 N = 1 kgm/s2 Weight force W newton N 9.80665 N = 1 kgf Time t second s SI base unit Velocity v metre per second m/s Acceleration a metre per second per second m/s2 Frequency (cycles per second) f hertz Hz 1 Hz = 1 c/s Bending moment (torque) M newton metre Nm Pressure P, F newton per square metre Pa (N/m²) 1 MN/m² = 1 N/mm² Stress σ (sigma) newton per square metre Pa (N/m²) Work, energy W joule J 1 J = 1 Nm Power P watt W 1 W = 1 J/s Quantity of heat Q joule J Thermodynamic temperature T kelvin K SI base unit Specific heat capacity c joule per kilogram degree kelvin J/ kg × K Thermal conductivity k watt per metre degree kelvin W/m × K Coefficient of heat U watt per square metre kelvin w/ m² × K 464 Rural structures in the tropics: design and development 3 MUltiples AND SUB MUltiples OF SI–UNITS COMMONLY USED IN CONSTRUCTION THEORY Factor Prefix Symbol 106 mega M 103 kilo k (102 hecto h) (10 deca da) (10-1 deci d) (10-2 centi c) 10-3 milli m 10-6 micro u Prefix in brackets should be avoided. -
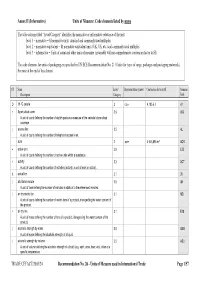
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

Beyond the Metre (Part II)
Iowa Science Teachers Journal Volume 17 Number 2 Article 10 1980 Beyond the Metre (Part II) Richard S. Thompkins Mississippi Bend Area Education Agency Vincent N. Lunetta University of Iowa Follow this and additional works at: https://scholarworks.uni.edu/istj Part of the Science and Mathematics Education Commons Let us know how access to this document benefits ouy Copyright © Copyright 1980 by the Iowa Academy of Science Recommended Citation Thompkins, Richard S. and Lunetta, Vincent N. (1980) "Beyond the Metre (Part II)," Iowa Science Teachers Journal: Vol. 17 : No. 2 , Article 10. Available at: https://scholarworks.uni.edu/istj/vol17/iss2/10 This Article is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Iowa Science Teachers Journal by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. BEYOND THE METRE (Part II) Richard S. Tompkins Metric Education Consultant Mississippi Bend Area Education Agency 2604 West Locust Street Davenport, Iowa 52804 Vincent N. Lunetta Associate Professor of Science Education University of Iowa Iowa City, Iowa 52242 Introduction This article is the second in a series discussing the wide spectrum of metric units. The first article dealt with some general aspects of the International System of Units (SI) and then went on to consider the metre, square metre, cubic metre and second. This article describes the SI unit of mass, and how units for velocity and acceleration are built using physical laws. The Metre Per Second (mis) Speed is usually represented in terms of a distance traveled during some time interval. -

Units of Measurement to Be Used in Air and Ground Operations
International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices,see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Suzanne TRANSMITTAL NOTE NEW EDITIONS OF ANNEXES TO THE CONVENTION ON INTERNATIONAL CIVIL AVIATION It has come to our attention that when a new edition of an Annex is published, users have been discarding, along with the previous edition of the Annex, the Supplement to the previous edition. Please note that the Supplement to the previous edition should be retained until a new Supplement is issued. Suzanne International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations ________________________________ This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices, see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Published in separate English, Arabic, Chinese, French, Russian and Spanish editions by the INTERNATIONAL CIVIL AVIATION ORGANIZATION 999 University Street, Montréal, Quebec, Canada H3C 5H7 For ordering information and for a complete listing of sales agents and booksellers, please go to the ICAO website at www.icao.int First edition 1948 Fourth edition 1979 Fifth edition 2010 Annex 5, Units of Measurement to be Used in Air and Ground Operations Order Number: AN 5 ISBN 978-92-9231-512-2 © ICAO 2010 All rights reserved. -

Chapter 2 Introduction Additional Resources
SCIENTIFIC MEASUREMENT AND DIMENSIONAL ANALYSIS Chapter 2 Memorise Understand Importance * SI units and prefixes * Dimensional analysis High level: 11% of GAMSAT Section 3 maths-based questions released by ACER are related to content in this chapter (in our estimation). * Note that approximately 90% of such questions are related to just 3 chapters: 1, 2, and 3. Introduction It is extremely important to know the SI system of measurement for the GAMSAT. The metric system is very much related to the SI system. The British system, though familiar, does not need to be memorised for the GAMSAT (related questions would only be asked if relevant conversion factors were provided). 'Dimensional analysis' is a technique whereby simply paying attention to units and applying the basic algebra we covered in GM Chapter 1, you will be able to solve several real exam questions in GAMSAT Physics, Chemistry and Biology – even without previous knowledge in those subjects. Additional Resources Open Discussion Boards Special Guest GAMSAT MATHS GM-37 2.0 Yes, GAMSAT Has a Need for Speed! A ‘unit’ is any standard used for Physics easier. It is normal if you cannot fill making comparisons in measurements. out the tables now, but please return to this Units form an important part of the founda- page after you have studied this chapter so tion of science and GAMSAT Section 3. you can complete all entries below. For non-science students, this is a Science students? Do your best, be new language to learn but the vocabulary quick, note that some entries that appear is incredibly small. -

Units of Measurement to Be Used in Air and Ground Operations
Manual of Standards - Units of Measurement to be used in Air and Ground Operations Version 1.1 : 18 November 2010 published by Civil Aviation Authority of Singapore INTENTIONALLY LEFT BLANK MOS – Units of Measurement to be used in Air and Ground Operations Table of Contents TABLE OF CONTENTS AMENDMENT RECORDS ii FOREWORD ii Chapter 1 Definitions 1-1 Chapter 2 Applicability 2-1 Chapter 3 Standard Application of Units of Measurement 3-1 Chapter 4 Termination of use of Non-SI Units 4-1 List of Tables Table 3-1: SI unit prefixes 3-1 Table 3-2: Non-SI units for use with the SI 3-2 Table 3-3: Non-SI units permitted for temporary use with the SI 3-3 Table 3-4: Standard application of specific units of measurement 3-3 Table 3-5: Temperature conversion formulae 3-7 Table 4-1: Termination dates for non-SI units 4-1 Version 1.1 : 18 November 2010 i MOS – Units of Measurement to be used in Air and Ground Operations Amendment Records AMENDMENT RECORDS The amendments listed below have been incorporated into this copy of the Manual of Standards – Units of Measurement to be used in Air and Ground Operations. Amendment Version Subject Source Sections affected Entered by Approved by Effective no. no. date (Date) (Date) - 1.0 Original version - All AAR Chief Executive 1 July 2010 (1 July 2010) (1 July 2010) 1 1.1 Unit for wind speed ICAO Annex 5 4.16 in Table 3-4 AAR Chief Executive 18 Nov 2010 (incorporating (1 Nov 2010) (1 Nov 2010) Amendment 17 applicable on 18 Nov 2010) Version 1.1 : 18 November 2010 ii MOS – Units of Measurement to be used in Air and Ground Operations Foreword FOREWORD Pursuant to paragraph 88(C) of the Air Navigation Order (ANO), this Manual of Standards – Units of Measurement to be used in Air and Ground Operations is issued by CAAS to specify the use of a standardized system of units of measurement in international civil aviation air and ground operations. -
Units and Conversions the Metric System Originates Back to the 1700S in France
Numeracy Introduction to Units and Conversions The metric system originates back to the 1700s in France. It is known as a decimal system because conversions between units are based on powers of ten. This is quite different to the Imperial system of units where every conversion has a unique value. A physical quantity is an attribute or property of a substance that can be expressed in a mathematical equation. A quantity, for example the amount of mass of a substance, is made up of a value and a unit. If a person has a mass of 72kg: the quantity being measured is Mass, the value of the measurement is 72 and the unit of measure is kilograms (kg). Another quantity is length (distance), for example the length of a piece of timber is 3.15m: the quantity being measured is length, the value of the measurement is 3.15 and the unit of measure is metres (m). A unit of measurement refers to a particular physical quantity. A metre describes length, a kilogram describes mass, a second describes time etc. A unit is defined and adopted by convention, in other words, everyone agrees that the unit will be a particular quantity. Historically, the metre was defined by the French Academy of Sciences as the length between two marks on a platinum-iridium bar at 0°C, which was designed to represent one ten-millionth of the distance from the Equator to the North Pole through Paris. In 1983, the metre was redefined as the distance travelled by light 1 in free space in of a second. -

Implementing a Physical Units Library for C++
Implementing a Physical Units Library for C++ Mateusz Pusz April 12, 2019 Famous motivating example The Mars Climate Orbiter • Robotic space probe launched by NASA on December 11, 1998 ACCU 2019 | Implementing a Physical Units Library for C++ 3 The Mars Climate Orbiter • Robotic space probe launched by NASA on December 11, 1998 • Project costs: $327.6 million – spacecra development: $193.1 million – launching it: $91.7 million – mission operations: $42.8 million ACCU 2019 | Implementing a Physical Units Library for C++ 3 The Mars Climate Orbiter • Robotic space probe launched by NASA on December 11, 1998 • Project costs: $327.6 million – spacecra development: $193.1 million – launching it: $91.7 million – mission operations: $42.8 million • Mars Climate Orbiter began the planned orbital insertion maneuver on September 23, 1999 at 09:00:46 UTC ACCU 2019 | Implementing a Physical Units Library for C++ 3 The Mars Climate Orbiter • Space probe went out of radio contact when it passed behind Mars at 09:04:52 UTC, 49 seconds earlier than expected • Communication was never reestablished • The spacecra disintegrated due to atmospheric stresses ACCU 2019 | Implementing a Physical Units Library for C++ 6 What went wrong? ACCU 2019 | Implementing a Physical Units Library for C++ 7 What went wrong? • The primary cause of this discrepancy was that – one piece of ground soware supplied by Lockheed Martin produced results in a United States customary unit, contrary to its Soware Interface Specification (SIS) – second system, supplied by NASA, expected -
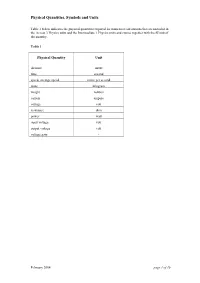
Quantity Symbol Sheet
Physical Quantities, Symbols and Units Table 1 below indicates the physical quantities required for numerical calculations that are included in the Access 3 Physics units and the Intermediate 1 Physics units and course together with the SI unit of the quantity. Table 1 Physical Quantity Unit distance metre time second speed, average speed metre per second mass kilogram weight newton current ampere voltage volt resistance ohm power watt input voltage volt output voltage volt voltage gain - February 2004 page 1 of 10 Physical Quantities, Symbols and Units Table 2 below indicates the physical quantities required for numerical calculations that are included in the Standard Grade Physics course together with: • the symbol used by SQA • the SI unit of the quantity (and alternative units included in the course) • the abbreviation for the unit used in Credit level examinations. In General level examinations full words are used for the units of all physical quantities. Table 2 Physical Quantity Symbol Unit Unit Abbreviation distance d or s metre m light year height h metre m wavelength λ metre m amplitude Α metre m time t second s speed, final speed v metre per second m/s initial speed u metre per second m/s change of speed ∆v metre per second m/s average speed v metre per second m/s frequency f hertz Hz acceleration a metre per second per second m/s2 acceleration due to gravity g metre per second per second m/s2 gravitational field strength g newton per kilogram N/kg mass m kilogram kg weight W newton N force, thrust F newton N energy E joule -
SI QUICK REFERENCE GUIDE: International System of Units (SI) the Modernized Metric System*
SI QUICK REFERENCE GUIDE: International System of Units (SI) The Modernized Metric System* UNITS The International System of Units (SI) is based on seven fundamental (base) units: Base Units Quantity Name Symbol length metre m mass kilogram kg time second s electric current ampere A thermodynamic temperature kelvin K amount of substance mole mol luminous intensity candela cd and a number of derived units which are combinations of base units and which may have special names and symbols: Examples of Derived Units Quantity Expression Name Symbol acceleration angular rad/s2 linear m/s2 angle plane dimensionless radian rad solid dimensionless steradian sr area m2 Celsius temperature K degree Celsius °C density heat flux W/ m2 mass kg/m3 current A/m2 energy, enthalpy work, heat N • m joule J specific J/ kg entropy heat capacity J/ K specific J/ (kg•K) flow, mass kg/s flow, volume m3/s force kg • m/s2 newton N frequency periodic 1/s hertz Hz rotating rev/s inductance Wb/A henry H magnetic flux V • s weber Wb mass flow kg/s moment of a force N • m potential,electric W/A volt V power, radiant flux J/s watt W pressure, stress N/m2 pascal Pa resistance, electric V/A ohm Ω thermal conductivity W/(m • K) velocity angular rad/s linear m/s viscosity dynamic (absolute)(µ)Pa• s kinematic (ν)m2/s volume m3 volume, specific m3/kg *For complete information see IEEE/ASTM SI-10. SI QUICK REFERENCE GUIDE SYMBOLS Symbol Name Quantity Formula A ampere electric current base unit Bq becquerel activity (of a radio nuclide) 1/s C coulomb electric charge A • s °C