Beyond the Metre (Part II)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M Motion Intervention Booklet.Pdf (810.7KB)
Motion Intervention Booklet 1 Random uncertainties have no pattern. They can cause a reading to be higher or lower than the “true value”. Systematic uncertainties have a pattern. They cause readings to be consistently too high or consistently too low. 2 The uncertainty in repeat readings is found from “half the range” and is given to 1 significant figure. Time / s Trial 1 Trial 2 Trial 3 Mean 1.23 1.20 1.28 Mean time = 1.23 + 1.20 + 1.28 = 1.23666666 => 1.24 (original data is to 2dp, so 3 the mean is to 2 dp as well) Range = 1.20 1.28. Uncertainty is ½ × (1.28 – 1.20) = ½ × 0.08 = ± 0.04 s 3 The speed at which a person can walk, run or cycle depends on many factors including: age, terrain, fitness and distance travelled. The speed of a moving object usually changes while it’s moving. Typical values for speeds may be taken as: walking: 1.5 m/s running: 3 m/s cycling: 6 m/s sound in air: 330 m/s 4 Scalar quantities have magnitude only. Speed is a scaler, eg speed = 30 m/s. Vector quantities have magnitude and direction. Velocity is a vector, eg velocity = 30 m/s north. Distance is how far something moves, it doesn’t involve direction. The displacement at a point is how far something is from the start point, in a straight line, including the direction. It doesn’t make any difference how far the object has moved in order to get to that point. -

CAR-ANS Part 5 Governing Units of Measurement to Be Used in Air and Ground Operations
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila UNCOTROLLED COPY INTENTIONALLY LEFT BLANK UNCOTROLLED COPY CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 UNCOTROLLED COPY CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

General Principles of Airborne Gravity Gradiometers for Mineral Exploration, in R.J.L
Geoscience Australia Record 2004/18 Airborne Gravity 2004 Abstracts from the ASEG-PESA Airborne Gravity 2004 Workshop Edited by Richard Lane Department of Industry, Tourism & Resources Minister for Industry, Tourism & Resources: The Hon. Ian Macfarlane, MP Parliamentary Secretary: The Hon. Warren Entsch, MP Secretary: Mark Paterson Geoscience Australia Chief Executive Officer: Neil Williams © Commonwealth of Australia 2004 This work is copyright. Apart from any fair dealings for the purposes of study, research, criticism or review, as permitted under the Copyright Act, no part may be reproduced by any process without written permission. Inquiries should be directed to the Communications Unit, Geoscience Australia, GPO Box 378, Canberra City, ACT, 2601 ISSN: 1448-2177 ISBN: 1 920871 13 6 GeoCat no. 61129 Bibliographic Reference: a) For the entire publication Lane, R.J.L., editor, 2004, Airborne Gravity 2004 – Abstracts from the ASEG-PESA Airborne Gravity 2004 Workshop: Geoscience Australia Record 2004/18. b) For an individual paper van Kann, F., 2004, Requirements and general principles of airborne gravity gradiometers for mineral exploration, in R.J.L. Lane, editor, Airborne Gravity 2004 - Abstracts from the ASEG-PESA Airborne Gravity 2004 Workshop: Geoscience Australia Record 2004/18, 1-5. Geoscience Australia has tried to make the information in this product as accurate as possible. However, it does not guarantee that the information is totally accurate or complete. THEREFORE, YOU SHOULD NOT RELY SOLELY ON THIS INFORMATION WHEN MAKING A COMMERCIAL DECISION. ii Contents “Airborne Gravity 2004 Workshop” Record R.J.L. Lane, M.H. Dransfield, D. Robson, R.J. Smith and G. Walker…………………………………..v Requirements and general principles of airborne gravity gradiometers for mineral exploration F. -

CAR-ANS PART 05 Issue No. 2 Units of Measurement to Be Used In
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila INTENTIONALLY LEFT BLANK CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -
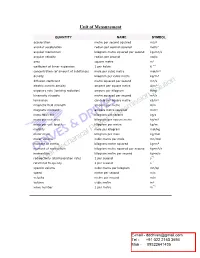
Unit of Measurement
Unit of Measurement QUANTITY NAME SYMBOL acceleration metre per second squared m/s² angular acceleration radian per second squared rad/s² angular momentum kilogram metre squared per second kg•m²/s angular velocity radian per second rad/s area square metre m² cœfficient of linear expansion 1 per kelvin K ¯¹ concentration (of amount of substance) mole per cubic metre mol/m³ density kilogram per cubic metre kg/m³ diffusion cœfficient metre squared per second m²/s electric current density ampere per square metre A/m² exposure rate (ionising radiation) ampere per kilogram A/kg kinematic viscosity metre squared per second m²/s luminance candela per square metre cd/m² magnetic field strength ampere per metre A/m magnetic moment ampere metre squared A•m² mass flow rate kilogram per second kg/s mass per unit area kilogram per square metre kg/m² mass per unit length kilogram per metre kg/m molality mole per kilogram mol/kg molar mass kilogram per mole kg/mol molar volume cubic metre per mole m³/mol moment of inertia kilogram metre squared kg•m² moment of momentum kilogram metre squared per second kg•m²/s momentum kilogram metre per second kg•m/s radioactivity (disintergration rate) 1 per second s¯¹ rotational frequency 1 per second s¯¹ specific volume cubic metre per kilogram m³/kg speed metre per second m/s velocity metre per second m/s volume cubic metre m³ wave number 1 per metre m¯¹ SI units and Additional QUANTITY NAME SYMBOL units absorbed dose joule per kilogram J/kg m²•s¯² watt per meter squared coefficient of heat transfer W/m²•K kg•s¯³•K¯¹ -

SI Base Units
463 Appendix I SI base units 1 THE SEVEN BASE UNITS IN THE INTERNatioNAL SYSTEM OF UNITS (SI) Quantity Name of Symbol base SI Unit Length metre m Mass kilogram kg Time second s Electric current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd 2 SOME DERIVED SI UNITS WITH THEIR SYMBOL/DerivatioN Quantity Common Unit Symbol Derivation symbol Term Term Length a, b, c metre m SI base unit Area A square metre m² Volume V cubic metre m³ Mass m kilogram kg SI base unit Density r (rho) kilogram per cubic metre kg/m³ Force F newton N 1 N = 1 kgm/s2 Weight force W newton N 9.80665 N = 1 kgf Time t second s SI base unit Velocity v metre per second m/s Acceleration a metre per second per second m/s2 Frequency (cycles per second) f hertz Hz 1 Hz = 1 c/s Bending moment (torque) M newton metre Nm Pressure P, F newton per square metre Pa (N/m²) 1 MN/m² = 1 N/mm² Stress σ (sigma) newton per square metre Pa (N/m²) Work, energy W joule J 1 J = 1 Nm Power P watt W 1 W = 1 J/s Quantity of heat Q joule J Thermodynamic temperature T kelvin K SI base unit Specific heat capacity c joule per kilogram degree kelvin J/ kg × K Thermal conductivity k watt per metre degree kelvin W/m × K Coefficient of heat U watt per square metre kelvin w/ m² × K 464 Rural structures in the tropics: design and development 3 MUltiples AND SUB MUltiples OF SI–UNITS COMMONLY USED IN CONSTRUCTION THEORY Factor Prefix Symbol 106 mega M 103 kilo k (102 hecto h) (10 deca da) (10-1 deci d) (10-2 centi c) 10-3 milli m 10-6 micro u Prefix in brackets should be avoided. -

SI QUICK REFERENCE GUIDE: International System of Units (SI) the Modernized Metric System*
SI QUICK REFERENCE GUIDE: International System of Units (SI) The Modernized Metric System* UNITS The International System of Units (SI) is based on seven fundamental (base) units: Base Units Quantity Name Symbol length metre m mass kilogram kg time second s electric current ampere A thermodynamic temperature kelvin K amount of substance mole mol luminous intensity candela cd and a number of derived units which are combinations of base units and which may have special names and symbols: Examples of Derived Units Quantity Expression Name Symbol acceleration angular rad/s2 linear m/s2 angle plane dimensionless radian rad solid dimensionless steradian sr area m2 Celsius temperature K degree Celsius °C density heat flux W/ m2 mass kg/m3 current A/m2 energy, enthalpy work, heat N • m joule J specific J/ kg entropy heat capacity J/ K specific J/ (kg•K) flow, mass kg/s flow, volume m3/s force kg • m/s2 newton N frequency periodic 1/s hertz Hz rotating rev/s inductance Wb/A henry H magnetic flux V • s weber Wb mass flow kg/s moment of a force N • m potential,electric W/A volt V power, radiant flux J/s watt W pressure, stress N/m2 pascal Pa resistance, electric V/A ohm Ω thermal conductivity W/(m • K) velocity angular rad/s linear m/s viscosity dynamic (absolute)(µ)Pa• s kinematic (ν)m2/s volume m3 volume, specific m3/kg *For complete information see IEEE/ASTM SI-10. SI QUICK REFERENCE GUIDE SYMBOLS Symbol Name Quantity Formula A ampere electric current base unit Bq becquerel activity (of a radio nuclide) 1/s C coulomb electric charge A • s °C -
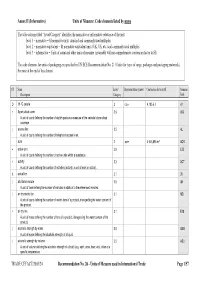
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

The International System of Units (SI) in Oceanography; Unesco Technical
Unesco technical papers in marine science 45 IAPSO Publication Scientifique No. 32 The International System of Units (SI) in Oceanography Report of IAPSO Working Group on Symbols, Units and Nomenclature in Physical Oceanography (SUN) This report was prepared under the auspices of the Inter national Association for the Physical Sciences of the Ocean (IAPSO). The first part is a revised version of IAPSO Publication Scientifique No. 31 (SUN Report). Unesco 1985 ' - i Published in 1985 by the United Nations Educational, Scientific and Cultural Organization, Place de Fontenoy, 75700 Paris. Printed in Unesco’s workshops. © Unesco 1985 Printed in France Reproduction authorized, providing that appropriate mention is made of Unesco Technical Papers in Marine Science and voucher copies are sent to the Division of Marine Sciences. PREFACE This series, the Unesco Technical Papers in Marine Science, is produced by the Unesco Division of Marine Sciences as a means of informing the scientific community of recent developments in oceanographic research and marine science affairs. Many of the texts published within the series result from research activities of the Scientific Committee on Oceanic Research (SCOR) and are submitted to Unesco for printing following final approval by SCOR of the relevant working group report. Unesco Technical Papers in Marine Science are distributed free of charge to various institutions and governmental authorities. Requests for copies of individual titles or additions to the mailing list should be addressed, on letterhead stationery if possible, to : Division of Marine Sciences, Unesco, Place de Fontenoy, 75700 Paris, France. XVI GENERAL ASSEMBLY OF THE INTERNATIONAL ASSOCIATION FOR THE PHYSICAL SCIENCES OF THE OCEAN (IAPSO) GRENOBLE, FRANCE, AUGUST 1975 RESOLUTION No 6 - 1975 IAPSO recommends the use of SI units and symbols in physical oceanography. -

Units of Measurement to Be Used in Air and Ground Operations
International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices,see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Suzanne TRANSMITTAL NOTE NEW EDITIONS OF ANNEXES TO THE CONVENTION ON INTERNATIONAL CIVIL AVIATION It has come to our attention that when a new edition of an Annex is published, users have been discarding, along with the previous edition of the Annex, the Supplement to the previous edition. Please note that the Supplement to the previous edition should be retained until a new Supplement is issued. Suzanne International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations ________________________________ This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices, see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Published in separate English, Arabic, Chinese, French, Russian and Spanish editions by the INTERNATIONAL CIVIL AVIATION ORGANIZATION 999 University Street, Montréal, Quebec, Canada H3C 5H7 For ordering information and for a complete listing of sales agents and booksellers, please go to the ICAO website at www.icao.int First edition 1948 Fourth edition 1979 Fifth edition 2010 Annex 5, Units of Measurement to be Used in Air and Ground Operations Order Number: AN 5 ISBN 978-92-9231-512-2 © ICAO 2010 All rights reserved. -

Chapter 2 Introduction Additional Resources
SCIENTIFIC MEASUREMENT AND DIMENSIONAL ANALYSIS Chapter 2 Memorise Understand Importance * SI units and prefixes * Dimensional analysis High level: 11% of GAMSAT Section 3 maths-based questions released by ACER are related to content in this chapter (in our estimation). * Note that approximately 90% of such questions are related to just 3 chapters: 1, 2, and 3. Introduction It is extremely important to know the SI system of measurement for the GAMSAT. The metric system is very much related to the SI system. The British system, though familiar, does not need to be memorised for the GAMSAT (related questions would only be asked if relevant conversion factors were provided). 'Dimensional analysis' is a technique whereby simply paying attention to units and applying the basic algebra we covered in GM Chapter 1, you will be able to solve several real exam questions in GAMSAT Physics, Chemistry and Biology – even without previous knowledge in those subjects. Additional Resources Open Discussion Boards Special Guest GAMSAT MATHS GM-37 2.0 Yes, GAMSAT Has a Need for Speed! A ‘unit’ is any standard used for Physics easier. It is normal if you cannot fill making comparisons in measurements. out the tables now, but please return to this Units form an important part of the founda- page after you have studied this chapter so tion of science and GAMSAT Section 3. you can complete all entries below. For non-science students, this is a Science students? Do your best, be new language to learn but the vocabulary quick, note that some entries that appear is incredibly small. -

Units of Measurement to Be Used in Air and Ground Operations
Manual of Standards - Units of Measurement to be used in Air and Ground Operations Version 1.1 : 18 November 2010 published by Civil Aviation Authority of Singapore INTENTIONALLY LEFT BLANK MOS – Units of Measurement to be used in Air and Ground Operations Table of Contents TABLE OF CONTENTS AMENDMENT RECORDS ii FOREWORD ii Chapter 1 Definitions 1-1 Chapter 2 Applicability 2-1 Chapter 3 Standard Application of Units of Measurement 3-1 Chapter 4 Termination of use of Non-SI Units 4-1 List of Tables Table 3-1: SI unit prefixes 3-1 Table 3-2: Non-SI units for use with the SI 3-2 Table 3-3: Non-SI units permitted for temporary use with the SI 3-3 Table 3-4: Standard application of specific units of measurement 3-3 Table 3-5: Temperature conversion formulae 3-7 Table 4-1: Termination dates for non-SI units 4-1 Version 1.1 : 18 November 2010 i MOS – Units of Measurement to be used in Air and Ground Operations Amendment Records AMENDMENT RECORDS The amendments listed below have been incorporated into this copy of the Manual of Standards – Units of Measurement to be used in Air and Ground Operations. Amendment Version Subject Source Sections affected Entered by Approved by Effective no. no. date (Date) (Date) - 1.0 Original version - All AAR Chief Executive 1 July 2010 (1 July 2010) (1 July 2010) 1 1.1 Unit for wind speed ICAO Annex 5 4.16 in Table 3-4 AAR Chief Executive 18 Nov 2010 (incorporating (1 Nov 2010) (1 Nov 2010) Amendment 17 applicable on 18 Nov 2010) Version 1.1 : 18 November 2010 ii MOS – Units of Measurement to be used in Air and Ground Operations Foreword FOREWORD Pursuant to paragraph 88(C) of the Air Navigation Order (ANO), this Manual of Standards – Units of Measurement to be used in Air and Ground Operations is issued by CAAS to specify the use of a standardized system of units of measurement in international civil aviation air and ground operations.