SI QUICK REFERENCE GUIDE: International System of Units (SI) the Modernized Metric System*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M Motion Intervention Booklet.Pdf (810.7KB)
Motion Intervention Booklet 1 Random uncertainties have no pattern. They can cause a reading to be higher or lower than the “true value”. Systematic uncertainties have a pattern. They cause readings to be consistently too high or consistently too low. 2 The uncertainty in repeat readings is found from “half the range” and is given to 1 significant figure. Time / s Trial 1 Trial 2 Trial 3 Mean 1.23 1.20 1.28 Mean time = 1.23 + 1.20 + 1.28 = 1.23666666 => 1.24 (original data is to 2dp, so 3 the mean is to 2 dp as well) Range = 1.20 1.28. Uncertainty is ½ × (1.28 – 1.20) = ½ × 0.08 = ± 0.04 s 3 The speed at which a person can walk, run or cycle depends on many factors including: age, terrain, fitness and distance travelled. The speed of a moving object usually changes while it’s moving. Typical values for speeds may be taken as: walking: 1.5 m/s running: 3 m/s cycling: 6 m/s sound in air: 330 m/s 4 Scalar quantities have magnitude only. Speed is a scaler, eg speed = 30 m/s. Vector quantities have magnitude and direction. Velocity is a vector, eg velocity = 30 m/s north. Distance is how far something moves, it doesn’t involve direction. The displacement at a point is how far something is from the start point, in a straight line, including the direction. It doesn’t make any difference how far the object has moved in order to get to that point. -

A Concurrent PASCAL Compiler for Minicomputers
512 Appendix A DIFFERENCES BETWEEN UCSD'S PASCAL AND STANDARD PASCAL The PASCAL language used in this book contains most of the features described by K. Jensen and N. Wirth in PASCAL User Manual and Report, Springer Verlag, 1975. We refer to the PASCAL defined by Jensen and Wirth as "Standard" PASCAL, because of its widespread acceptance even though no international standard for the language has yet been established. The PASCAL used in this book has been implemented at University of California San Diego (UCSD) in a complete software system for use on a variety of small stand-alone microcomputers. This will be referred to as "UCSD PASCAL", which differs from the standard by a small number of omissions, a very small number of alterations, and several extensions. This appendix provides a very brief summary Of these differences. Only the PASCAL constructs used within this book will be mentioned herein. Documents are available from the author's group at UCSD describing UCSD PASCAL in detail. 1. CASE Statements Jensen & Wirth state that if there is no label equal to the value of the case statement selector, then the result of the case statement is undefined. UCSD PASCAL treats this situation by leaving the case statement normally with no action being taken. 2. Comments In UCSD PASCAL, a comment appears between the delimiting symbols "(*" and "*)". If the opening delimiter is followed immediately by a dollar sign, as in "(*$", then the remainder of the comment is treated as a directive to the compiler. The only compiler directive mentioned in this book is (*$G+*), which tells the compiler to allow the use of GOTO statements. -

Illumination and Distance
PHYS 1400: Physical Science Laboratory Manual ILLUMINATION AND DISTANCE INTRODUCTION How bright is that light? You know, from experience, that a 100W light bulb is brighter than a 60W bulb. The wattage measures the energy used by the bulb, which depends on the bulb, not on where the person observing it is located. But you also know that how bright the light looks does depend on how far away it is. That 100W bulb is still emitting the same amount of energy every second, but if you are farther away from it, the energy is spread out over a greater area. You receive less energy, and perceive the light as less bright. But because the light energy is spread out over an area, it’s not a linear relationship. When you double the distance, the energy is spread out over four times as much area. If you triple the distance, the area is nine Twice the distance, ¼ as bright. Triple the distance? 11% as bright. times as great, meaning that you receive only 1/9 (or 11%) as much energy from the light source. To quantify the amount of light, we will use units called lux. The idea is simple: energy emitted per second (Watts), spread out over an area (square meters). However, a lux is not a W/m2! A lux is a lumen per m2. So, what is a lumen? Technically, it’s one candela emitted uniformly across a solid angle of 1 steradian. That’s not helping, is it? Examine the figure above. The source emits light (energy) in all directions simultaneously. -

2.1 Definition of the SI
CCPR/16-53 Modifications to the Draft of the ninth SI Brochure dated 16 September 2016 recommended by the CCPR to the CCU via the CCPR president Takashi Usuda, Wednesday 14 December 2016. The text in black is a selection of paragraphs from the brochure with the section title for indication. The sentences to be modified appear in red. 2.1 Definition of the SI Like for any value of a quantity, the value of a fundamental constant can be expressed as the product of a number and a unit as Q = {Q} [Q]. The definitions below specify the exact numerical value of each constant when its value is expressed in the corresponding SI unit. By fixing the exact numerical value the unit becomes defined, since the product of the numerical value {Q} and the unit [Q] has to equal the value Q of the constant, which is postulated to be invariant. The seven constants are chosen in such a way that any unit of the SI can be written either through a defining constant itself or through products or ratios of defining constants. The International System of Units, the SI, is the system of units in which the unperturbed ground state hyperfine splitting frequency of the caesium 133 atom Cs is 9 192 631 770 Hz, the speed of light in vacuum c is 299 792 458 m/s, the Planck constant h is 6.626 070 040 ×1034 J s, the elementary charge e is 1.602 176 620 8 ×1019 C, the Boltzmann constant k is 1.380 648 52 ×1023 J/K, 23 -1 the Avogadro constant NA is 6.022 140 857 ×10 mol , 12 the luminous efficacy of monochromatic radiation of frequency 540 ×10 hertz Kcd is 683 lm/W. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Recommended Light Levels
Recommended Light Levels Recommended Light Levels (Illuminance) for Outdoor and Indoor Venues This is an instructor resource with information to be provided to students as the instructor sees fit. Light Level or Illuminance, is the amount of light measured in a plane surface (or the total luminous flux incident on a surface, per unit area). The work plane is where the most important tasks in the room or space are performed. Measuring Units of Light Level - Illuminance Illuminance is measured in foot candles (ftcd, fc, fcd) or lux (in the metric SI system). A foot candle is actually one lumen of light density per square foot; one lux is one lumen per square meter. • 1 lux = 1 lumen / sq meter = 0.0001 phot = 0.0929 foot candle (ftcd, fcd) • 1 phot = 1 lumen / sq centimeter = 10000 lumens / sq meter = 10000 lux • 1 foot candle (ftcd, fcd) = 1 lumen / sq ft = 10.752 lux Common Light Levels Outdoors from Natural Sources Common light levels outdoor at day and night can be found in the table below: Illumination Condition (ftcd) (lux) Sunlight 10,000 107,527 Full Daylight 1,000 10,752 Overcast Day 100 1,075 Very Dark Day 10 107 Twilight 1 10.8 Deep Twilight .1 1.08 Full Moon .01 .108 Quarter Moon .001 .0108 Starlight .0001 .0011 Overcast Night .00001 .0001 Common Light Levels Outdoors from Manufactured Sources The nomenclature for most of the types of areas listed in the table below can be found in the City of Los Angeles, Department of Public Works, Bureau of Street Lighting’s “DESIGN STANDARDS AND GUIDELINES” at the URL address under References at the end of this document. -
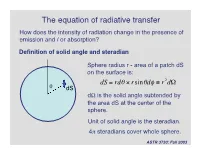
The Equation of Radiative Transfer How Does the Intensity of Radiation Change in the Presence of Emission and / Or Absorption?
The equation of radiative transfer How does the intensity of radiation change in the presence of emission and / or absorption? Definition of solid angle and steradian Sphere radius r - area of a patch dS on the surface is: dS = rdq ¥ rsinqdf ≡ r2dW q dS dW is the solid angle subtended by the area dS at the center of the † sphere. Unit of solid angle is the steradian. 4p steradians cover whole sphere. ASTR 3730: Fall 2003 Definition of the specific intensity Construct an area dA normal to a light ray, and consider all the rays that pass through dA whose directions lie within a small solid angle dW. Solid angle dW dA The amount of energy passing through dA and into dW in time dt in frequency range dn is: dE = In dAdtdndW Specific intensity of the radiation. † ASTR 3730: Fall 2003 Compare with definition of the flux: specific intensity is very similar except it depends upon direction and frequency as well as location. Units of specific intensity are: erg s-1 cm-2 Hz-1 steradian-1 Same as Fn Another, more intuitive name for the specific intensity is brightness. ASTR 3730: Fall 2003 Simple relation between the flux and the specific intensity: Consider a small area dA, with light rays passing through it at all angles to the normal to the surface n: n o In If q = 90 , then light rays in that direction contribute zero net flux through area dA. q For rays at angle q, foreshortening reduces the effective area by a factor of cos(q). -

Application Note to the Field Pumping Non-Newtonian Fluids with Liquiflo Gear Pumps
Pumping Non-Newtonian Fluids Application Note to the Field with Liquiflo Gear Pumps Application Note Number: 0104-2 Date: April 10, 2001; Revised Jan. 2016 Newtonian vs. non-Newtonian Fluids: Fluids fall into one of two categories: Newtonian or non-Newtonian. A Newtonian fluid has a constant viscosity at a particular temperature and pressure and is independent of shear rate. A non-Newtonian fluid has viscosity that varies with shear rate. The apparent viscosity is a measure of the resistance to flow of a non-Newtonian fluid at a given temperature, pressure and shear rate. Newton’s Law states that shear stress () is equal the dynamic viscosity () multiplied by the shear rate (): = . A fluid which obeys this relationship, where is constant, is called a Newtonian fluid. Therefore, for a Newtonian fluid, shear stress is directly proportional to shear rate. If however, varies as a function of shear rate, the fluid is non-Newtonian. In the SI system, the unit of shear stress is pascals (Pa = N/m2), the unit of shear rate is hertz or reciprocal seconds (Hz = 1/s), and the unit of dynamic viscosity is pascal-seconds (Pa-s). In the cgs system, the unit of shear stress is dynes per square centimeter (dyn/cm2), the unit of shear rate is again hertz or reciprocal seconds, and the unit of dynamic viscosity is poises (P = dyn-s-cm-2). To convert the viscosity units from one system to another, the following relationship is used: 1 cP = 1 mPa-s. Pump shaft speed is normally measured in RPM (rev/min). -

The Decibel Scale R.C
The Decibel Scale R.C. Maher Fall 2014 It is often convenient to compare two quantities in an audio system using a proportionality ratio. For example, if a linear amplifier produces 2 volts (V) output amplitude when its input amplitude is 100 millivolts (mV), the voltage gain is expressed as the ratio of output/input: 2V/100mV = 20. As long as the two quantities being compared have the same units--volts in this case--the proportionality ratio is dimensionless. If the proportionality ratios of interest end up being very large or very small, such as 2x105 and 2.5x10-4, manipulating and interpreting the results can become rather unwieldy. In this situation it can be helpful to compress the numerical range by taking the logarithm of the ratio. It is customary to use a base-10 logarithm for this purpose. For example, 5 log10(2x10 ) = 5 + log10(2) = 5.301 and -4 log10(2.5x10 ) = -4 + log10(2.5) = -3.602 If the quantities in the proportionality ratio both have the units of power (e.g., 2 watts), or intensity (watts/m ), then the base-10 logarithm log10(power1/power0) is expressed with the unit bel (symbol: B), in honor of Alexander Graham Bell (1847 -1922). The decibel is a unit representing one tenth (deci-) of a bel. Therefore, a figure reported in decibels is ten times the value reported in bels. The expression for a proportionality ratio expressed in decibel units (symbol dB) is: 10 푝표푤푒푟1 10 Common Usage 푑퐵 ≡ ∙ 푙표푔 �푝표푤푒푟0� The power dissipated in a resistance R ohms can be expressed as V2/R, where V is the voltage across the resistor. -

Relationships of the SI Derived Units with Special Names and Symbols and the SI Base Units
Relationships of the SI derived units with special names and symbols and the SI base units Derived units SI BASE UNITS without special SI DERIVED UNITS WITH SPECIAL NAMES AND SYMBOLS names Solid lines indicate multiplication, broken lines indicate division kilogram kg newton (kg·m/s2) pascal (N/m2) gray (J/kg) sievert (J/kg) 3 N Pa Gy Sv MASS m FORCE PRESSURE, ABSORBED DOSE VOLUME STRESS DOSE EQUIVALENT meter m 2 m joule (N·m) watt (J/s) becquerel (1/s) hertz (1/s) LENGTH J W Bq Hz AREA ENERGY, WORK, POWER, ACTIVITY FREQUENCY second QUANTITY OF HEAT HEAT FLOW RATE (OF A RADIONUCLIDE) s m/s TIME VELOCITY katal (mol/s) weber (V·s) henry (Wb/A) tesla (Wb/m2) kat Wb H T 2 mole m/s CATALYTIC MAGNETIC INDUCTANCE MAGNETIC mol ACTIVITY FLUX FLUX DENSITY ACCELERATION AMOUNT OF SUBSTANCE coulomb (A·s) volt (W/A) C V ampere A ELECTRIC POTENTIAL, CHARGE ELECTROMOTIVE ELECTRIC CURRENT FORCE degree (K) farad (C/V) ohm (V/A) siemens (1/W) kelvin Celsius °C F W S K CELSIUS CAPACITANCE RESISTANCE CONDUCTANCE THERMODYNAMIC TEMPERATURE TEMPERATURE t/°C = T /K – 273.15 candela 2 steradian radian cd lux (lm/m ) lumen (cd·sr) 2 2 (m/m = 1) lx lm sr (m /m = 1) rad LUMINOUS INTENSITY ILLUMINANCE LUMINOUS SOLID ANGLE PLANE ANGLE FLUX The diagram above shows graphically how the 22 SI derived units with special names and symbols are related to the seven SI base units. In the first column, the symbols of the SI base units are shown in rectangles, with the name of the unit shown toward the upper left of the rectangle and the name of the associated base quantity shown in italic type below the rectangle. -

Basic Vacuum Theory
MIDWEST TUNGSTEN Tips SERVICE BASIC VACUUM THEORY Published, now and again by MIDWEST TUNGSTEN SERVICE Vacuum means “emptiness” in Latin. It is defi ned as a space from which all air and other gases have been removed. This is an ideal condition. The perfect vacuum does not exist so far as we know. Even in the depths of outer space there is roughly one particle (atom or molecule) per cubic centimeter of space. Vacuum can be used in many different ways. It can be used as a force to hold things in place - suction cups. It can be used to move things, as vacuum cleaners, drinking straws, and siphons do. At one time, part of the train system of England was run using vacuum technology. The term “vacuum” is also used to describe pressures that are subatmospheric. These subatmospheric pressures range over 19 orders of magnitude. Vacuum Ranges Ultra high vacuum Very high High Medium Low _________________________|_________|_____________|__________________|____________ _ 10-16 10-14 10-12 10-10 10-8 10-6 10-4 10-3 10-2 10-1 1 10 102 103 (pressure in torr) There is air pressure, or atmospheric pressure, all around and within us. We use a barometer to measure this pressure. Torricelli built the fi rst mercury barometer in 1644. He chose the pressure exerted by one millimeter of mercury at 0ºC in the tube of the barometer to be his unit of measure. For many years millimeters of mercury (mmHg) have been used as a standard unit of pressure. One millimeter of mercury is known as one Torr in honor of Torricelli.