SI Base Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M Motion Intervention Booklet.Pdf (810.7KB)
Motion Intervention Booklet 1 Random uncertainties have no pattern. They can cause a reading to be higher or lower than the “true value”. Systematic uncertainties have a pattern. They cause readings to be consistently too high or consistently too low. 2 The uncertainty in repeat readings is found from “half the range” and is given to 1 significant figure. Time / s Trial 1 Trial 2 Trial 3 Mean 1.23 1.20 1.28 Mean time = 1.23 + 1.20 + 1.28 = 1.23666666 => 1.24 (original data is to 2dp, so 3 the mean is to 2 dp as well) Range = 1.20 1.28. Uncertainty is ½ × (1.28 – 1.20) = ½ × 0.08 = ± 0.04 s 3 The speed at which a person can walk, run or cycle depends on many factors including: age, terrain, fitness and distance travelled. The speed of a moving object usually changes while it’s moving. Typical values for speeds may be taken as: walking: 1.5 m/s running: 3 m/s cycling: 6 m/s sound in air: 330 m/s 4 Scalar quantities have magnitude only. Speed is a scaler, eg speed = 30 m/s. Vector quantities have magnitude and direction. Velocity is a vector, eg velocity = 30 m/s north. Distance is how far something moves, it doesn’t involve direction. The displacement at a point is how far something is from the start point, in a straight line, including the direction. It doesn’t make any difference how far the object has moved in order to get to that point. -

8.1 Basic Terms & Conversions in the Metric System 1/1000 X Base Unit M
___________________________________ 8.1 Basic Terms & Conversions in the Metric System ___________________________________ Basic Units of Measurement: •Meter (m) used to measure length. A little longer than a yard. ___________________________________ ___________________________________ •Kilogram (kg) used to measure mass. A little more than 2 pounds. ___________________________________ •Liter (l) used to measure volume. A little more than a quart. •Celsius (°C) used to measure temperature. ___________________________________ ___________________________________ ch. 8 Angel & Porter (6th ed.) 1 The metric system is based on powers of 10 (the decimal system). ___________________________________ Prefix Symbol Meaning ___________________________________ kilo k 1000 x base unit ___________________________________ hecto h 100 x base unit deka da 10 x base unit ___________________________________ ——— ——— base unit deci d 1/10 x base unit ___________________________________ centi c 1/100 x base unit milli m 1/1000 x base unit ___________________________________ ___________________________________ ch. 8 Angel & Porter (6th ed.) 2 ___________________________________ Changing Units within the Metric System 1. To change from a smaller unit to a larger unit, move the ___________________________________ decimal point in the original quantity one place to the for each larger unit of measurement until you obtain the desired unit of measurement. ___________________________________ 2. To change form a larger unit to a smaller unit, move the decimal point -

A Learner's Guide to SI Units and Their Conversion
A Learner's Guide to SI Units and their Conversion October 2004 Workbook for students Learner's Guide to SI Units and their Conversion their and Units SI to Guide Learner's Edexcel A London Qualifications is one of the leading examining and awarding bodies in the UK and throughout the world. It incorporates all the qualifications previously awarded under the Edexcel and BTEC brand. We provide a wide range of qualifications including general (academic), vocational, occupational and specific programmes for employers. Through a network of UK and overseas offices, our centres receive the support they need to help them deliver their education and training programmes to learners. For further information please call Customer Services on 0870 240 9800, or visit our website at www.edexcel.org.uk Authorised by Jim Dobson Prepared by Sarah Harrison All the material in this publication is copyright © London Qualifications Limited 2004 CONTENTS Introduction 1 What are units? 2 Operations with units 5 Submultiple and multiple units 9 Conversion of units 11 Conversion examples and exercises 13 Length 13 Area 14 Volume 15 Mass 16 Time 17 Temperature 18 Density 19 Force 20 Stress and pressure 21 Answers to exercises 23 Introduction One of the important areas where Science and Technology students need support is in the conversion of units. This booklet is designed to be useful for students in all Science, Technology and Engineering subjects. This booklet has been produced to: S introduce students to SI base and derived units and S help students with the conversion of multiple and sub-multiple units to SI base and derived units. -

Lesson 1: Length English Vs
Lesson 1: Length English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer B. 1 yard or 1 meter C. 1 inch or 1 centimeter English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers 1 yard = 0.9444 meters English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers 1 inch = 2.54 centimeters 1 yard = 0.9444 meters Metric Units The basic unit of length in the metric system in the meter and is represented by a lowercase m. Standard: The distance traveled by light in absolute vacuum in 1∕299,792,458 of a second. Metric Units 1 Kilometer (km) = 1000 meters 1 Meter = 100 Centimeters (cm) 1 Meter = 1000 Millimeters (mm) Which is larger? A. 1 meter or 105 centimeters C. 12 centimeters or 102 millimeters B. 4 kilometers or 4400 meters D. 1200 millimeters or 1 meter Measuring Length How many millimeters are in 1 centimeter? 1 centimeter = 10 millimeters What is the length of the line in centimeters? _______cm What is the length of the line in millimeters? _______mm What is the length of the line to the nearest centimeter? ________cm HINT: Round to the nearest centimeter – no decimals. -

Lord Kelvin and the Age of the Earth.Pdf
ME201/MTH281/ME400/CHE400 Lord Kelvin and the Age of the Earth Lord Kelvin (1824 - 1907) 1. About Lord Kelvin Lord Kelvin was born William Thomson in Belfast Ireland in 1824. He attended Glasgow University from the age of 10, and later took his BA at Cambridge. He was appointed Professor of Natural Philosophy at Glasgow in 1846, a position he retained the rest of his life. He worked on a broad range of topics in physics, including thermody- namics, electricity and magnetism, hydrodynamics, atomic physics, and earth science. He also had a strong interest in practical problems, and in 1866 he was knighted for his work on the transtlantic cable. In 1892 he became Baron Kelvin, and this name survives as the name of the absolute temperature scale which he proposed in 1848. During his long career, Kelvin published more than 600 papers. He was elected to the Royal Society in 1851, and served as president of that organization from 1890 to 1895. The information in this section and the picture above were taken from a very useful web site called the MacTu- tor History of Mathematics Archive, sponsored by St. Andrews University. The web address is http://www-history.mcs.st-and.ac.uk/~history/ 2 kelvin.nb 2. The Age of the Earth The earth shows it age in many ways. Some techniques for estimating this age require us to observe the present state of a time-dependent process, and from that observation infer the time at which the process started. If we believe that the process started when the earth was formed, we get an estimate of the earth's age. -

The Avogadro Constant to Be Equal to Exactly 6.02214X×10 23 When It Is Expressed in the Unit Mol −1
[august, 2011] redefinition of the mole and the new system of units zoltan mester It is as easy to count atomies as to resolve the propositions of a lover.. As You Like It William Shakespeare 1564-1616 Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, German Empire, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States and Venezuela 1875 -May 20 1875, BIPM, CGPM and the CIPM was established, and a three- dimensional mechanical unit system was setup with the base units metre, kilogram, and second. -1901 Giorgi showed that it is possible to combine the mechanical units of this metre–kilogram–second system with the practical electric units to form a single coherent four-dimensional system -In 1921 Consultative Committee for Electricity (CCE, now CCEM) -by the 7th CGPM in 1927. The CCE to proposed, in 1939, the adoption of a four-dimensional system based on the metre, kilogram, second, and ampere, the MKSA system, a proposal approved by the ClPM in 1946. -In 1954, the 10th CGPM, the introduction of the ampere, the kelvin and the candela as base units -in 1960, 11th CGPM gave the name International System of Units, with the abbreviation SI. -in 1970, the 14 th CGMP introduced mole as a unit of amount of substance to the SI 1960 Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Much improved atomic weight estimates, oxygen = 100 . Jöns Jacob Berzelius (1779–1848) Further improved atomic weight estimates . -

Page 1 of 29 Dalton Transactions
Dalton Transactions Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/dalton Page 1 of 29 Dalton Transactions Core-level photoemission spectra of Mo 0.3 Cu 0.7 Sr 2ErCu 2Oy, a superconducting perovskite derivative. Unconventional structure/property relations a, b b b a Sourav Marik , Christine Labrugere , O.Toulemonde , Emilio Morán , M. A. Alario- Manuscript Franco a,* aDpto. Química Inorgánica, Facultad de CC.Químicas, Universidad Complutense de Madrid, 28040- Madrid (Spain) bCNRS, Université de Bordeaux, ICMCB, 87 avenue du Dr. A. Schweitzer, Pessac, F-33608, France -

Natural Units Conversions and Fundamental Constants James D
February 2, 2016 Natural Units Conversions and Fundamental Constants James D. Wells Michigan Center for Theoretical Physics (MCTP) University of Michigan, Ann Arbor Abstract: Conversions are listed between basis units of natural units system where ~ = c = 1. Important fundamental constants are given in various equivalent natural units based on GeV, seconds, and meters. Natural units basic conversions Natural units are defined to give ~ = c = 1. All quantities with units then can be written in terms of a single base unit. It is customary in high-energy physics to use the base unit GeV. But it can be helpful to think about the equivalences in terms of other base units, such as seconds, meters and even femtobarns. The conversion factors are based on various combinations of ~ and c (Olive 2014). For example −25 1 = ~ = 6:58211928(15) × 10 GeV s; and (1) 1 = c = 2:99792458 × 108 m s−1: (2) From this we can derive several useful basic conversion factors and 1 = ~c = 0:197327 GeV fm; and (3) 2 11 2 1 = (~c) = 3:89379 × 10 GeV fb (4) where I have not included the error in ~c conversion but if needed can be obtained by consulting the error in ~. Note, the value of c has no error since it serves to define the meter, which is the distance light travels in vacuum in 1=299792458 of a second (Olive 2014). The unit fb is a femtobarn, which is 10−15 barns. A barn is defined to be 1 barn = 10−24 cm2. The prefexes letters, such as p on pb, etc., mean to multiply the unit after it by the appropropriate power: femto (f) 10−15, pico (p) 10−12, nano (n) 10−9, micro (µ) 10−6, milli (m) 10−3, kilo (k) 103, mega (M) 106, giga (G) 109, terra (T) 1012, and peta (P) 1015. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

2019 Redefinition of SI Base Units
2019 redefinition of SI base units A redefinition of SI base units is scheduled to come into force on 20 May 2019.[1][2] The kilogram, ampere, kelvin, and mole will then be defined by setting exact numerical values for the Planck constant (h), the elementary electric charge (e), the Boltzmann constant (k), and the Avogadro constant (NA), respectively. The metre and candela are already defined by physical constants, subject to correction to their present definitions. The new definitions aim to improve the SI without changing the size of any units, thus ensuring continuity with existing measurements.[3][4] In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes,[5][6] which the International Committee for Weights and Measures (CIPM) had proposed earlier that year.[7]:23 The previous major change of the metric system was in 1960 when the International System of Units (SI) was formally published. The SI is a coherent system structured around seven base units whose definitions are unconstrained by that of any other unit and another twenty-two named units derived from these base units. The metre was redefined in terms of the wavelength of a spectral line of a The SI system after the 2019 redefinition: krypton-86 radiation,[Note 1] making it derivable from universal natural Dependence of base unit definitions onphysical constants with fixed numerical values and on other phenomena, but the kilogram remained defined in terms of a physical prototype, base units. leaving it the only artefact upon which the SI unit definitions depend. The metric system was originally conceived as a system of measurement that was derivable from unchanging phenomena,[8] but practical limitations necessitated the use of artefacts (the prototype metre and prototype kilogram) when the metric system was first introduced in France in 1799. -
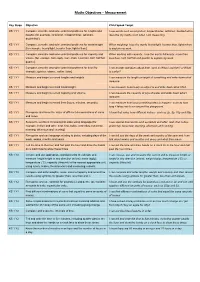
Maths Objectives – Measurement
Maths Objectives – Measurement Key Stage Objective Child Speak Target KS 1 Y1 Compare, describe and solve practical problems for lengths and I use words such as long/short, longer/shorter, tall/short, double/half to heights [for example, long/short, longer/shorter, tall/short, describe my maths work when I am measuring. double/half]. KS 1 Y1 Compare, describe and solve practical problems for mass/weight When weighing, I use the words heavy/light, heavier than, lighter than [for example, heavy/light, heavier than, lighter than]. to explain my work. KS 1 Y1 Compare, describe and solve practical problems for capacity and When working with capacity, I use the words full/empty, more than, volume [for example, full/empty, more than, less than, half, half full, less than, half, half full and quarter to explain my work. quarter]. KS 1 Y1 Compare, describe and solve practical problems for time [for I can answer questions about time, such as Who is quicker? or What example, quicker, slower, earlier, later]. is earlier? KS 1 Y1 Measure and begin to record lengths and heights. I can measure the length or height of something and write down what measure. KS 1 Y1 Measure and begin to record mass/weight. I can measure how heavy an object is and write down what I find. KS 1 Y1 Measure and begin to record capacity and volume. I can measure the capacity of jugs of water and write down what I measure. KS 1 Y1 Measure and begin to record time (hours, minutes, seconds). I can measure how long something takes to happen - such as how long it takes me to run around the playground. -

Etir Code Lists
eTIR Code Lists Code lists CL01 Equipment size and type description code (UN/EDIFACT 8155) Code specifying the size and type of equipment. 1 Dime coated tank A tank coated with dime. 2 Epoxy coated tank A tank coated with epoxy. 6 Pressurized tank A tank capable of holding pressurized goods. 7 Refrigerated tank A tank capable of keeping goods refrigerated. 9 Stainless steel tank A tank made of stainless steel. 10 Nonworking reefer container 40 ft A 40 foot refrigerated container that is not actively controlling temperature of the product. 12 Europallet 80 x 120 cm. 13 Scandinavian pallet 100 x 120 cm. 14 Trailer Non self-propelled vehicle designed for the carriage of cargo so that it can be towed by a motor vehicle. 15 Nonworking reefer container 20 ft A 20 foot refrigerated container that is not actively controlling temperature of the product. 16 Exchangeable pallet Standard pallet exchangeable following international convention. 17 Semi-trailer Non self propelled vehicle without front wheels designed for the carriage of cargo and provided with a kingpin. 18 Tank container 20 feet A tank container with a length of 20 feet. 19 Tank container 30 feet A tank container with a length of 30 feet. 20 Tank container 40 feet A tank container with a length of 40 feet. 21 Container IC 20 feet A container owned by InterContainer, a European railway subsidiary, with a length of 20 feet. 22 Container IC 30 feet A container owned by InterContainer, a European railway subsidiary, with a length of 30 feet. 23 Container IC 40 feet A container owned by InterContainer, a European railway subsidiary, with a length of 40 feet.