White Light Is a Mixture of Many Wavelengths (Colors) and Retinal Cells React Differently to Different Wavelengths. How the Huma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 LIGHT PHYSICS Light and Lighting Francesco Anselmo Light Intro Light Animates and Reveals Architecture
1 LIGHT PHYSICS Light and Lighting Francesco Anselmo Light intro Light animates and reveals architecture. Architecture cannot fully exist without light, since without light there would be nothing to see. Yet in architectural design light is usually either expected from nature or developed as an add-on attachment very late in the design process. The course explores the symbiotic relationship between architecture and light. As much as light can reveal architecture, architecture can animate light, making it bounce, scatter, refract, altering its spectrum and colour perception, absorbing it or reflecting it, modulating its path and strength in both space and time. It aims at developing a sensibility and intuition to the qualities of light, whilst giving the physical and computational tools to explore and validate design ideas. 4 7 1 2 5 3 6 1 LIGHT PHYSICS 4 LIGHT ELECTRIC 7 LIGHT CONNECTED 2 LIGHT BIOLOGY 5 LIGHT ARCHITECTURE 3 LIGHT NATURAL 6 LIGHT VIRTUAL Reading list Books Free online resources Bachelard, Gaston. The poetics of space, Beacon Press 1992 • http://hyperphysics.phy-astr.gsu.edu/hbase/ligcon.html Banham, Reyner. The architecture of the well-tempered Environment, Chicago University Press 1984 • http://thedaylightsite.com/ Bazerman, Charles. The languages of Edison’s light, MIT Press 2002 Berger, John. Ways of seeing, Pearson Education, Limited, 2002 • http://issuu.com/lightonline/docs/handbook-of-lighting-design Berger, John. About looking, Bloomsbury Publishing 2009 • http://www.radiance-online.org/ Bluhm, Andreas. Light! The industrial age 1750-1900, Carnegie Museum of Art 2000 Boyce, Peter R. Human Factors in Lighting, Taylor & Francis 2003 Calvino, Italo. -

Download the DIA Color Chart
DEMI-PERMANENT HAIRCOLOR 2 COMPLIMENTARY LINES OF HAIRCOLOR Each with its own benefit & expertise both provide maximum creativity & freedom ADVANCED ALKALINE TECHNOLOGY GENTLE ACID TECHNOLOGY HIGH PERFORMANCE HIGH PERFORMANCE • Demi-permanent crème • Luminous demi-permanent gel-crème • Rich tones, exceptional softness • Zero lift • Covers up to 70% grey • Intense care for the hair THE PROCESS THE PROCESS • Lifts (up to 1.5 Levels with 15-vol), then deposits • Zero lift, deposit only BEFORE COLOR DURING COLOR AFTER COLOR BEFORE COLOR DURING COLOR AFTER COLOR On natural hair, the cuticle The alkaline agents slightly open DIA Richesse has the ability to lighten up On color-treated & DIA Light has an acid The cationic polymers in scales are closed. the hair fiber allowing colorants to 1.5 levels, cover up to 70% white hair sensitized hair, the cuticle pH close to the natural pH of the hair. DIA Light have a resurfacing to penetrate the cuticle. & create rich, profound tones. scales are already open. effect on the cuticle, leaving There is no lift with gentle penetration the hair with amazing shine. of colorants for long lasting color. ADVANCED ALKALINE TECHNOLOGY ADVANCED ALKALINE TECHNOLOGY HIGH PERFORMANCE • Demi-permanent crème • Rich tones, exceptional softness • Covers up to 70% grey THE PROCESS • Lifts (up to 1.5 Levels with 15-vol), then deposits BEFORE COLOR DURING COLOR AFTER COLOR On natural hair, the cuticle The alkaline agents slightly open DIA Richesse has the ability to lighten scales are closed. the hair fiber allowing colorants up to 1.5 levels, cover up to 70% white to penetrate the cuticle. -

White by Law---Haney Lopez (Abridged Version)
White by Law The Legal Construction of Race Revised and Updated 10th Anniversary Edition Ian Haney Lόpez NEW YORK UNIVERSITY PRESS New York and London (2006) 1│White Lines In its first words on the subject of citizenship, Congress in 1790 restricted naturalization to “white persons.” Though the requirements for naturalization changed frequently thereafter, this racial prerequisite to citizenship endured for over a century and a half, remaining in force until 1952. From the earliest years of this country until just a generation ago, being a “white person” was a condition for acquiring citizenship. Whether one was “white” however, was often no easy question. As immigration reached record highs at the turn of this century, countless people found themselves arguing their racial identity in order to naturalize. From 1907, when the federal government began collecting data on naturalization, until 1920, over one million people gained citizenship under the racially restrictive naturalization laws. Many more sought to naturalize and were rejected. Naturalization rarely involved formal court proceedings and therefore usually generated few if any written records beyond the simple decision. However, a number of cases construing the “white person” prerequisite reached the highest state and federal judicial circles, and two were argued before the U.S. Supreme Court in the early 1920s. These cases produced illuminating published decisions that document the efforts of would-be citizens from around the world to establish their Whiteness at law. Applicants from Hawaii, China, Japan, Burma, and the Philippines, as well as all mixed- race applicants, failed in their arguments. Conversely, courts ruled that applicants from Mexico and Armenia were “white,” but vacillated over the Whiteness of petitioners from Syria, India, and Arabia. -

WHITE LIGHT and COLORED LIGHT Grades K–5
WHITE LIGHT AND COLORED LIGHT grades K–5 Objective This activity offers two simple ways to demonstrate that white light is made of different colors of light mixed together. The first uses special glasses to reveal the colors that make up white light. The second involves spinning a colorful top to blend different colors into white. Together, these activities can be thought of as taking white light apart and putting it back together again. Introduction The Sun, the stars, and a light bulb are all sources of “white” light. But what is white light? What we see as white light is actually a combination of all visible colors of light mixed together. Astronomers spread starlight into a rainbow or spectrum to study the specific colors of light it contains. The colors hidden in white starlight can reveal what the star is made of and how hot it is. The tool astronomers use to spread light into a spectrum is called a spectroscope. But many things, such as glass prisms and water droplets, can also separate white light into a rainbow of colors. After it rains, there are often lots of water droplets in the air. White sunlight passing through these droplets is spread apart into its component colors, creating a rainbow. In this activity, you will view the rainbow of colors contained in white light by using a pair of “Rainbow Glasses” that separate white light into a spectrum. ! SAFETY NOTE These glasses do NOT protect your eyes from the Sun. NEVER LOOK AT THE SUN! Background Reading for Educators Light: Its Secrets Revealed, available at http://www.amnh.org/education/resources/rfl/pdf/du_x01_light.pdf Developed with the generous support of The Charles Hayden Foundation WHITE LIGHT AND COLORED LIGHT Materials Rainbow Glasses Possible white light sources: (paper glasses containing a Incandescent light bulb diffraction grating). -

Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC
Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC Introduction Why does the color of a copper chloride solution appear blue? As the white light hits the paint, which colors does the solution absorb and which colors does it transmit? In this activity students will observe the basic principles of absorption spectroscopy based on absorbance and transmittance of visible light. Concepts • Spectroscopy • Visible light spectrum • Absorbance and transmittance • Quantized electron energy levels Background The visible light spectrum (380−750 nm) is the light we are able to see. This spectrum is often referred to as “ROY G BIV” as a mnemonic device for the order of colors it produces. Violet has the shortest wavelength (about 400 nm) and red has the longest wavelength (about 650–700 nm). Many common chemical solutions can be used as filters to demonstrate the principles of absorption and transmittance of visible light in the electromagnetic spectrum. For example, copper(II) chloride (blue), ammonium dichromate (orange), iron(III) chloride (yellow), and potassium permanganate (red) are all different colors because they absorb different wave- lengths of visible light. In this demonstration, students will observe the principles of absorption spectroscopy using a variety of different colored solutions. Food coloring will be substituted for the orange and yellow chemical solutions mentioned above. Rare earth metal solutions, erbium and praseodymium chloride, will be used to illustrate line absorption spectra. Materials Copper(II) chloride solution, 1 M, 85 mL Diffraction grating, holographic, 14 cm × 14 cm Erbium chloride solution, 0.1 M, 50 mL Microchemistry solution bottle, 50 mL, 6 Potassium permanganate solution (KMnO4), 0.001 M, 275 mL Overhead projector and screen Praseodymium chloride solution, 0.1 M, 50 mL Red food dye Water, deionized Stir rod, glass Beaker, 250-mL Tape Black construction paper, 12 × 18, 2 sheets Yellow food dye Colored pencils Safety Precautions Copper(II) chloride solution is toxic by ingestion and inhalation. -
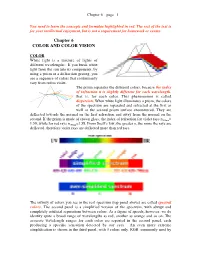
Chapter 6 COLOR and COLOR VISION
Chapter 6 – page 1 You need to learn the concepts and formulae highlighted in red. The rest of the text is for your intellectual enjoyment, but is not a requirement for homework or exams. Chapter 6 COLOR AND COLOR VISION COLOR White light is a mixture of lights of different wavelengths. If you break white light from the sun into its components, by using a prism or a diffraction grating, you see a sequence of colors that continuously vary from red to violet. The prism separates the different colors, because the index of refraction n is slightly different for each wavelength, that is, for each color. This phenomenon is called dispersion. When white light illuminates a prism, the colors of the spectrum are separated and refracted at the first as well as the second prism surface encountered. They are deflected towards the normal on the first refraction and away from the normal on the second. If the prism is made of crown glass, the index of refraction for violet rays n400nm= 1.59, while for red rays n700nm=1.58. From Snell’s law, the greater n, the more the rays are deflected, therefore violet rays are deflected more than red rays. The infinity of colors you see in the real spectrum (top panel above) are called spectral colors. The second panel is a simplified version of the spectrum, with abrupt and completely artificial separations between colors. As a figure of speech, however, we do identify quite a broad range of wavelengths as red, another as orange and so on. -

Light, Color, and Atmospheric Optics
Light, Color, and Atmospheric Optics GEOL 1350: Introduction To Meteorology 1 2 • During the scattering process, no energy is gained or lost, and therefore, no temperature changes occur. • Scattering depends on the size of objects, in particular on the ratio of object’s diameter vs wavelength: 1. Rayleigh scattering (D/ < 0.03) 2. Mie scattering (0.03 ≤ D/ < 32) 3. Geometric scattering (D/ ≥ 32) 3 4 • Gas scattering: redirection of radiation by a gas molecule without a net transfer of energy of the molecules • Rayleigh scattering: absorption extinction 4 coefficient s depends on 1/ . • Molecules scatter short (blue) wavelengths preferentially over long (red) wavelengths. • The longer pathway of light through the atmosphere the more shorter wavelengths are scattered. 5 • As sunlight enters the atmosphere, the shorter visible wavelengths of violet, blue and green are scattered more by atmospheric gases than are the longer wavelengths of yellow, orange, and especially red. • The scattered waves of violet, blue, and green strike the eye from all directions. • Because our eyes are more sensitive to blue light, these waves, viewed together, produce the sensation of blue coming from all around us. 6 • Rayleigh Scattering • The selective scattering of blue light by air molecules and very small particles can make distant mountains appear blue. The blue ridge mountains in Virginia. 7 • When small particles, such as fine dust and salt, become suspended in the atmosphere, the color of the sky begins to change from blue to milky white. • These particles are large enough to scatter all wavelengths of visible light fairly evenly in all directions. -

The Electromagnetic Spectrum
The Electromagnetic Spectrum Wavelength/frequency/energy MAP TAP 2003-2004 The Electromagnetic Spectrum 1 Teacher Page • Content: Physical Science—The Electromagnetic Spectrum • Grade Level: High School • Creator: Dorothy Walk • Curriculum Objectives: SC 1; Intro Phys/Chem IV.A (waves) MAP TAP 2003-2004 The Electromagnetic Spectrum 2 MAP TAP 2003-2004 The Electromagnetic Spectrum 3 What is it? • The electromagnetic spectrum is the complete spectrum or continuum of light including radio waves, infrared, visible light, ultraviolet light, X- rays and gamma rays • An electromagnetic wave consists of electric and magnetic fields which vibrates thus making waves. MAP TAP 2003-2004 The Electromagnetic Spectrum 4 Waves • Properties of waves include speed, frequency and wavelength • Speed (s), frequency (f) and wavelength (l) are related in the formula l x f = s • All light travels at a speed of 3 s 108 m/s in a vacuum MAP TAP 2003-2004 The Electromagnetic Spectrum 5 Wavelength, Frequency and Energy • Since all light travels at the same speed, wavelength and frequency have an indirect relationship. • Light with a short wavelength will have a high frequency and light with a long wavelength will have a low frequency. • Light with short wavelengths has high energy and long wavelength has low energy MAP TAP 2003-2004 The Electromagnetic Spectrum 6 MAP TAP 2003-2004 The Electromagnetic Spectrum 7 Radio waves • Low energy waves with long wavelengths • Includes FM, AM, radar and TV waves • Wavelengths of 10-1m and longer • Low frequency • Used in many -

Colour Vision Deficiency
Eye (2010) 24, 747–755 & 2010 Macmillan Publishers Limited All rights reserved 0950-222X/10 $32.00 www.nature.com/eye Colour vision MP Simunovic REVIEW deficiency Abstract effective "treatment" of colour vision deficiency: whilst it has been suggested that tinted lenses Colour vision deficiency is one of the could offer a means of enabling those with commonest disorders of vision and can be colour vision deficiency to make spectral divided into congenital and acquired forms. discriminations that would normally elude Congenital colour vision deficiency affects as them, clinical trials of such lenses have been many as 8% of males and 0.5% of femalesFthe largely disappointing. Recent developments in difference in prevalence reflects the fact that molecular genetics have enabled us to not only the commonest forms of congenital colour understand more completely the genetic basis of vision deficiency are inherited in an X-linked colour vision deficiency, they have opened the recessive manner. Until relatively recently, our possibility of gene therapy. The application of understanding of the pathophysiological basis gene therapy to animal models of colour vision of colour vision deficiency largely rested on deficiency has shown dramatic results; behavioural data; however, modern molecular furthermore, it has provided interesting insights genetic techniques have helped to elucidate its into the plasticity of the visual system with mechanisms. respect to extracting information about the The current management of congenital spectral composition of the visual scene. colour vision deficiency lies chiefly in appropriate counselling (including career counselling). Although visual aids may Materials and methods be of benefit to those with colour vision deficiency when performing certain tasks, the This article was prepared by performing a evidence suggests that they do not enable primary search of Pubmed for articles on wearers to obtain normal colour ‘colo(u)r vision deficiency’ and ‘colo(u)r discrimination. -

1 Human Color Vision
CAMC01 9/30/04 3:13 PM Page 1 1 Human Color Vision Color appearance models aim to extend basic colorimetry to the level of speci- fying the perceived color of stimuli in a wide variety of viewing conditions. To fully appreciate the formulation, implementation, and application of color appearance models, several fundamental topics in color science must first be understood. These are the topics of the first few chapters of this book. Since color appearance represents several of the dimensions of our visual experience, any system designed to predict correlates to these experiences must be based, to some degree, on the form and function of the human visual system. All of the color appearance models described in this book are derived with human visual function in mind. It becomes much simpler to understand the formulations of the various models if the basic anatomy, physiology, and performance of the visual system is understood. Thus, this book begins with a treatment of the human visual system. As necessitated by the limited scope available in a single chapter, this treatment of the visual system is an overview of the topics most important for an appreciation of color appearance modeling. The field of vision science is immense and fascinating. Readers are encouraged to explore the liter- ature and the many useful texts on human vision in order to gain further insight and details. Of particular note are the review paper on the mechan- isms of color vision by Lennie and D’Zmura (1988), the text on human color vision by Kaiser and Boynton (1996), the more general text on the founda- tions of vision by Wandell (1995), the comprehensive treatment by Palmer (1999), and edited collections on color vision by Backhaus et al. -

Color Vision Deficiency
Color Vision Deficiency What is color vision deficiency? Color vision deficiency is called “color blindness” by mistake. Actually, the term describes a number of different problems people have with color vision. Abnormal color vision may vary from not being able to tell certain colors apart to not being able to identify any color. Whom does color vision deficiency affect? An estimated 8% of males and fewer than 1% of females have color vision problems. Most color vision problems run in families and are inherited and present at birth. A child inherits a color vision deficiency by receiving a faulty color vision gene from a parent. Abnormal color vision is found in a recessive gene on the X chromosome. Men are born with just one X and one Y chromosome. However, women have two X chromosomes. Because of this, women can sometimes overcome the faulty gene with their second normal X chromosome. Men, unfortunately, do not have a second X chromosome to help compensate for the faulty color vision gene. Heredity does not cause all color vision problems. One common problem happens from the normal aging of the eye’s lens. The lens is clear at birth, but the aging process causes it to darken and yellow. Older adults may have problems identifying certain dark colors, particularly blues. Certain medications as well as inherited or acquired retinal and optic nerve disease, may also affect normal color vision. Who should be tested for color deficiency? Any child who is having difficulty in school should be checked for possible visual problems including color vision impairment. -

Electromagnetic Spectrum
Electromagnetic Spectrum Why do some things have colors? What makes color? Why do fast food restaurants use red lights to keep food warm? Why don’t they use green or blue light? Why do X-rays pass through the body and let us see through the body? What has the radio to do with radiation? What are the night vision devices that the army uses in night time fighting? To find the answers to these questions we have to examine the electromagnetic spectrum. FASTER THAN A SPEEDING BULLET MORE POWERFUL THAN A LOCOMOTIVE These words were used to introduce a fictional superhero named Superman. These same words can be used to help describe Electromagnetic Radiation. Electromagnetic Radiation is like a two member team racing together at incredible speeds across the vast regions of space or flying from the clutches of a tiny atom. They travel together in packages called photons. Moving along as a wave with frequency and wavelength they travel at the velocity of 186,000 miles per second (300,000,000 meters per second) in a vacuum. The photons are so tiny they cannot be seen even with powerful microscopes. If the photon encounters any charged particles along its journey it pushes and pulls them at the same frequency that the wave had when it started. The waves can circle the earth more than seven times in one second! If the waves are arranged in order of their wavelength and frequency the waves form the Electromagnetic Spectrum. They are described as electromagnetic because they are both electric and magnetic in nature.