Color Vision Mechanisms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter 6 COLOR and COLOR VISION
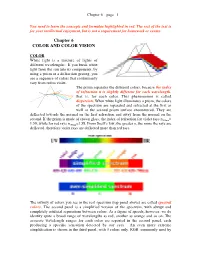
Chapter 6 – page 1 You need to learn the concepts and formulae highlighted in red. The rest of the text is for your intellectual enjoyment, but is not a requirement for homework or exams. Chapter 6 COLOR AND COLOR VISION COLOR White light is a mixture of lights of different wavelengths. If you break white light from the sun into its components, by using a prism or a diffraction grating, you see a sequence of colors that continuously vary from red to violet. The prism separates the different colors, because the index of refraction n is slightly different for each wavelength, that is, for each color. This phenomenon is called dispersion. When white light illuminates a prism, the colors of the spectrum are separated and refracted at the first as well as the second prism surface encountered. They are deflected towards the normal on the first refraction and away from the normal on the second. If the prism is made of crown glass, the index of refraction for violet rays n400nm= 1.59, while for red rays n700nm=1.58. From Snell’s law, the greater n, the more the rays are deflected, therefore violet rays are deflected more than red rays. The infinity of colors you see in the real spectrum (top panel above) are called spectral colors. The second panel is a simplified version of the spectrum, with abrupt and completely artificial separations between colors. As a figure of speech, however, we do identify quite a broad range of wavelengths as red, another as orange and so on. -

Hue and Saturation Shifts from Spatially Induced Blackness
Bimler et al. Vol. 26, No. 1/January 2009/J. Opt. Soc. Am. A 163 Hue and saturation shifts from spatially induced blackness David L. Bimler,1,* Galina V. Paramei,2 and Chingis A. Izmailov3 1Department of Health and Human Development, Massey University, Private Bag 11-222, Palmerston North, New Zealand 2Department of Psychology, Liverpool Hope University, Hope Park, L16 9JD Liverpool, United Kingdom 3Department of Psychophysiology, Moscow Lomonosov State University, Mokhovaya st. 11/5, 125009 Moscow, Russia *Corresponding author: [email protected] Received June 24, 2008; revised September 15, 2008; accepted September 21, 2008; posted November 3, 2008 (Doc. ID 97849); published December 24, 2008 We studied changes in the color appearance of a chromatic stimulus as it underwent simultaneous contrast with a more luminous surround. Three normal trichromats provided color-naming descriptions for a 10 cd/m2 monochromatic field while a broadband white annulus surround ranged in luminance from 0.2 cd/m2 to 200 cd/m2. Descriptions of the chromatic field included Red, Green, Blue, Yellow, White, and Black or their combinations. The naming frequencies for each color/surround were used to calculate measures of similarity among the stimuli. Multidimensional scaling analysis of these subjective similarities resulted in a four-dimensional color space with two chromatic axes, red–green and blue–yellow, and two achromatic axes, revealing separate qualities of blackness/lightness and saturation. Contrast-induced darkening of the chro- matic field was found to be accompanied by shifts in both hue and saturation. Hue shifts were similar to the Bezold–Brücke shift; shifts in saturation were also quantified. -

Colour Vision Deficiency
Eye (2010) 24, 747–755 & 2010 Macmillan Publishers Limited All rights reserved 0950-222X/10 $32.00 www.nature.com/eye Colour vision MP Simunovic REVIEW deficiency Abstract effective "treatment" of colour vision deficiency: whilst it has been suggested that tinted lenses Colour vision deficiency is one of the could offer a means of enabling those with commonest disorders of vision and can be colour vision deficiency to make spectral divided into congenital and acquired forms. discriminations that would normally elude Congenital colour vision deficiency affects as them, clinical trials of such lenses have been many as 8% of males and 0.5% of femalesFthe largely disappointing. Recent developments in difference in prevalence reflects the fact that molecular genetics have enabled us to not only the commonest forms of congenital colour understand more completely the genetic basis of vision deficiency are inherited in an X-linked colour vision deficiency, they have opened the recessive manner. Until relatively recently, our possibility of gene therapy. The application of understanding of the pathophysiological basis gene therapy to animal models of colour vision of colour vision deficiency largely rested on deficiency has shown dramatic results; behavioural data; however, modern molecular furthermore, it has provided interesting insights genetic techniques have helped to elucidate its into the plasticity of the visual system with mechanisms. respect to extracting information about the The current management of congenital spectral composition of the visual scene. colour vision deficiency lies chiefly in appropriate counselling (including career counselling). Although visual aids may Materials and methods be of benefit to those with colour vision deficiency when performing certain tasks, the This article was prepared by performing a evidence suggests that they do not enable primary search of Pubmed for articles on wearers to obtain normal colour ‘colo(u)r vision deficiency’ and ‘colo(u)r discrimination. -

1 Human Color Vision
CAMC01 9/30/04 3:13 PM Page 1 1 Human Color Vision Color appearance models aim to extend basic colorimetry to the level of speci- fying the perceived color of stimuli in a wide variety of viewing conditions. To fully appreciate the formulation, implementation, and application of color appearance models, several fundamental topics in color science must first be understood. These are the topics of the first few chapters of this book. Since color appearance represents several of the dimensions of our visual experience, any system designed to predict correlates to these experiences must be based, to some degree, on the form and function of the human visual system. All of the color appearance models described in this book are derived with human visual function in mind. It becomes much simpler to understand the formulations of the various models if the basic anatomy, physiology, and performance of the visual system is understood. Thus, this book begins with a treatment of the human visual system. As necessitated by the limited scope available in a single chapter, this treatment of the visual system is an overview of the topics most important for an appreciation of color appearance modeling. The field of vision science is immense and fascinating. Readers are encouraged to explore the liter- ature and the many useful texts on human vision in order to gain further insight and details. Of particular note are the review paper on the mechan- isms of color vision by Lennie and D’Zmura (1988), the text on human color vision by Kaiser and Boynton (1996), the more general text on the founda- tions of vision by Wandell (1995), the comprehensive treatment by Palmer (1999), and edited collections on color vision by Backhaus et al. -

Color Vision Deficiency
Color Vision Deficiency What is color vision deficiency? Color vision deficiency is called “color blindness” by mistake. Actually, the term describes a number of different problems people have with color vision. Abnormal color vision may vary from not being able to tell certain colors apart to not being able to identify any color. Whom does color vision deficiency affect? An estimated 8% of males and fewer than 1% of females have color vision problems. Most color vision problems run in families and are inherited and present at birth. A child inherits a color vision deficiency by receiving a faulty color vision gene from a parent. Abnormal color vision is found in a recessive gene on the X chromosome. Men are born with just one X and one Y chromosome. However, women have two X chromosomes. Because of this, women can sometimes overcome the faulty gene with their second normal X chromosome. Men, unfortunately, do not have a second X chromosome to help compensate for the faulty color vision gene. Heredity does not cause all color vision problems. One common problem happens from the normal aging of the eye’s lens. The lens is clear at birth, but the aging process causes it to darken and yellow. Older adults may have problems identifying certain dark colors, particularly blues. Certain medications as well as inherited or acquired retinal and optic nerve disease, may also affect normal color vision. Who should be tested for color deficiency? Any child who is having difficulty in school should be checked for possible visual problems including color vision impairment. -

Chromatic Function of the Cones D H Foster, University of Manchester, Manchester, UK
Chromatic Function of the Cones D H Foster, University of Manchester, Manchester, UK ã 2010 Elsevier Ltd. All rights reserved. Glossary length. If l is the path length and a(l) is the spectral absorptivity, then, for a homogeneous isotropic CIE, Commission Internationale de l’Eclairage – absorbing medium, A(l)=la(l) (Lambert’s law). The CIE is an independent, nonprofit organization Spectral absorptance a(l) – Ratio of the spectral responsible for the international coordination of radiant flux absorbed by a layer to the spectral lighting-related technical standards, including radiant flux entering the layer. If t(l) is the spectral colorimetry standards. transmittance, then a(l)=1Àt(l). The value of a(l) Color-matching functions – Functions of depends on the length or thickness of the layer. For a wavelength l that describe the amounts of three homogeneous isotropic absorbing medium, fixed primary lights which, when mixed, match a a(l)=1Àt(l)=1À10Àla(l), where l is the path length monochromatic light of wavelength l of constant and a(l) is the spectral absorptivity. Changes in the radiant power. The amounts may be negative. The concentration of a photopigment have the same color-matching functions obtained with any two effect as changes in path length. different sets of primaries are related by a linear Spectral absorptivity a(l) – Spectral absorbance of transformation. Particular sets of color-matching a layer of unit thickness. Absorptivity is a functions have been standardized by the CIE. characteristic of the medium, that is, the Fundamental spectral sensitivities – The color- photopigment. Its numerical value depends on the matching functions corresponding to the spectral unit of length. -

Cone Fundamentals and CIE Standards
Cone fundamentals and CIE standards Andrew Stockman UCL Institute of Ophthalmology, London, UK Address UCL Institute of Ophthalmology, 11-43 Bath Street, London EC1V 9EL, UK Corresponding author: Stockman, Andrew ([email protected]) Abstract A knowledge of the spectral sensitivities of the long- (L-), middle- (M-) and short- (S-) wavelength-sensitive cone types is vital for modelling human color vision and for the practical applications of color matching and color specification. After being agnostic about defining standard cone spectral sensitivities, the Commission Internationale de l' Éclairage (CIE) has sanctioned the cone spectral sensitivity estimates of Stockman & Sharpe [1] and the associated measures of luminous efficiency [2,3] as “physiologically-relevant” standards for color vision [4,5]. These can be used to model mean normal color vision at the photoreceptor level and postreceptorally. Both LMS and XYZ versions have been defined for 2-deg and 10-deg vision. Built into the standards are corrections for individual differences in macular and lens pigment densities, but individual differences in photopigment optical density and the spectral position of the cone photopigments can also be accommodated [6,7]. Understanding the CIE standard and its advantages is of current interest and importance. 1 Color perception and photopic visual function are inextricably linked to and, indeed, limited by the properties of the three cone photoceptors: the long- (L-), middle- (M-) and short- (S-) wavelength-sensitive cones. This short review covers the derivation of the recent “physiologically- relevant” Commission Internationale de l' Éclairage (CIE) 2006; 2015 cone spectral sensitivities and luminous efficiency functions for 2-deg and 10-deg vision [4,5] and provides background details about cone spectral sensitivities and trichromatic color matching. -

Color Vision
Color Vision Lecture 10 Chapter 5, Part A Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Princeton University, Fall 2017 1 Exam #1: Thursday 10/19 Format: multiple-choice, fill-in-the-blank, & short answer What to study: - all material from lectures & slides - precept readings (basic gist & findings of each article) (If something appeared only in the book, and not at all in class or precept or slides, you can probably safely ignore it) Review session: tonight @ 7:00 in PNI A30 2 2015 called…. Grey-and-green, or pink-and-white? 3 4 5 • color vision has evolutionary value • lack of color vision ≠ black & white 6 Basic Principles of Color Perception The book says: “Color is not a physical property but a psychophysical property” 7 Basic Principles of Color Perception • Most of the light we see is reflected • Typical light sources: Sun, light bulb, LED screen • We see only part of the electromagnetic spectrum(between 400 and 700 nm). Why?? 8 Basic Principles of Color Perception • Why only 400-700 nm? (Pomerantz, Rice U.) Suggestion: unique ability to penetrate sea water 9 Basic Principles of Color Perception Q: How many numbers would you need to write down to specify the spectral properties of a light source? A: It depends on how you “bin” up the spectrum • One number for each spectral “bin”: 20 17 16 15 13 12 10 example: 13 5 bins energy 0 0 0 0 0 10 Basic Principles of Color Perception Device: hyper-spectral camera - measures the amount of energy (or number of photons) in each small range of wavelengths - can use thousands of bins (or “frequency bands”) instead of just the 13 shown here 20 17 16 15 13 12 10 5 energy 0 0 0 0 0 11 Basic Principles of Color Perception Some terminology for colored light: spectral - referring to the wavelength of light the illuminant - light source power spectrum - this curve. -

Color Vision and Night Vision Chapter Dingcai Cao 10
Retinal Diagnostics Section 2 For additional online content visit http://www.expertconsult.com Color Vision and Night Vision Chapter Dingcai Cao 10 OVERVIEW ROD AND CONE FUNCTIONS Day vision and night vision are two separate modes of visual Differences in the anatomy and physiology (see Chapters 4, perception and the visual system shifts from one mode to the Autofluorescence imaging, and 9, Diagnostic ophthalmic ultra- other based on ambient light levels. Each mode is primarily sound) of the rod and cone systems underlie different visual mediated by one of two photoreceptor classes in the retina, i.e., functions and modes of visual perception. The rod photorecep- cones and rods. In day vision, visual perception is primarily tors are responsible for our exquisite sensitivity to light, operat- cone-mediated and perceptions are chromatic. In other words, ing over a 108 (100 millionfold) range of illumination from near color vision is present in the light levels of daytime. In night total darkness to daylight. Cones operate over a 1011 range of vision, visual perception is rod-mediated and perceptions are illumination, from moonlit night light levels to light levels that principally achromatic. Under dim illuminations, there is no are so high they bleach virtually all photopigments in the cones. obvious color vision and visual perceptions are graded varia- Together the rods and cones function over a 1014 range of illu- tions of light and dark. Historically, color vision has been studied mination. Depending on the relative activity of rods and cones, as the salient feature of day vision and there has been emphasis a light level can be characterized as photopic (cones alone on analysis of cone activities in color vision. -

Radiometry and Photometry
Radiometry and Photometry Wei-Chih Wang Department of Power Mechanical Engineering National TsingHua University W. Wang Materials Covered • Radiometry - Radiant Flux - Radiant Intensity - Irradiance - Radiance • Photometry - luminous Flux - luminous Intensity - Illuminance - luminance Conversion from radiometric and photometric W. Wang Radiometry Radiometry is the detection and measurement of light waves in the optical portion of the electromagnetic spectrum which is further divided into ultraviolet, visible, and infrared light. Example of a typical radiometer 3 W. Wang Photometry All light measurement is considered radiometry with photometry being a special subset of radiometry weighted for a typical human eye response. Example of a typical photometer 4 W. Wang Human Eyes Figure shows a schematic illustration of the human eye (Encyclopedia Britannica, 1994). The inside of the eyeball is clad by the retina, which is the light-sensitive part of the eye. The illustration also shows the fovea, a cone-rich central region of the retina which affords the high acuteness of central vision. Figure also shows the cell structure of the retina including the light-sensitive rod cells and cone cells. Also shown are the ganglion cells and nerve fibers that transmit the visual information to the brain. Rod cells are more abundant and more light sensitive than cone cells. Rods are 5 sensitive over the entire visible spectrum. W. Wang There are three types of cone cells, namely cone cells sensitive in the red, green, and blue spectral range. The approximate spectral sensitivity functions of the rods and three types or cones are shown in the figure above 6 W. Wang Eye sensitivity function The conversion between radiometric and photometric units is provided by the luminous efficiency function or eye sensitivity function, V(λ). -

The Eye and Night Vision
Source: http://www.aoa.org/x5352.xml Print This Page The Eye and Night Vision (This article has been adapted from the excellent USAF Special Report, AL-SR-1992-0002, "Night Vision Manual for the Flight Surgeon", written by Robert E. Miller II, Col, USAF, (RET) and Thomas J. Tredici, Col, USAF, (RET)) THE EYE The basic structure of the eye is shown in Figure 1. The anterior portion of the eye is essentially a lens system, made up of the cornea and crystalline lens, whose primary purpose is to focus light onto the retina. The retina contains receptor cells, rods and cones, which, when stimulated by light, send signals to the brain. These signals are subsequently interpreted as vision. Most of the receptors are rods, which are found predominately in the periphery of the retina, whereas the cones are located mostly in the center and near periphery of the retina. Although there are approximately 17 rods for every cone, the cones, concentrated centrally, allow resolution of fine detail and color discrimination. The rods cannot distinguish colors and have poor resolution, but they have a much higher sensitivity to light than the cones. DAY VERSUS NIGHT VISION According to a widely held theory of vision, the rods are responsible for vision under very dim levels of illumination (scotopic vision) and the cones function at higher illumination levels (photopic vision). Photopic vision provides the capability for seeing color and resolving fine detail (20/20 of better), but it functions only in good illumination. Scotopic vision is of poorer quality; it is limited by reduced resolution ( 20/200 or less) and provides the ability to discriminate only between shades of black and white. -

Color Appearance Models Second Edition
Color Appearance Models Second Edition Mark D. Fairchild Munsell Color Science Laboratory Rochester Institute of Technology, USA Color Appearance Models Wiley–IS&T Series in Imaging Science and Technology Series Editor: Michael A. Kriss Formerly of the Eastman Kodak Research Laboratories and the University of Rochester The Reproduction of Colour (6th Edition) R. W. G. Hunt Color Appearance Models (2nd Edition) Mark D. Fairchild Published in Association with the Society for Imaging Science and Technology Color Appearance Models Second Edition Mark D. Fairchild Munsell Color Science Laboratory Rochester Institute of Technology, USA Copyright © 2005 John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England Telephone (+44) 1243 779777 This book was previously publisher by Pearson Education, Inc Email (for orders and customer service enquiries): [email protected] Visit our Home Page on www.wileyeurope.com or www.wiley.com All Rights Reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except under the terms of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London W1T 4LP, UK, without the permission in writing of the Publisher. Requests to the Publisher should be addressed to the Permissions Department, John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, or emailed to [email protected], or faxed to (+44) 1243 770571. This publication is designed to offer Authors the opportunity to publish accurate and authoritative information in regard to the subject matter covered.