The Stille Reaction Chem 115
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proquest Dissertations
The synthesis of highly substituted indoles via isonitriles Item Type text; Dissertation-Reproduction (electronic) Authors Kennedy, Abigail Rose Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 26/09/2021 04:52:53 Link to Item http://hdl.handle.net/10150/290368 INFORMATION TO USERS This manuscript has l)een reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be firom any type of computer printer. The quality of this raproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author dkJ not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sectkms with small overiaps. Photographs included in the original manuscript have been reproduced xerographicaliy in this copy. Higher quality 6' x 9* black and white photographk: prints are available for any photographs or illustrations appearing in this copy for an additk)nai charge. -

Oxidative Addition & Reductive Elimination
3/1/2021 Oxidative Addition & Reductive Elimination Robert H. Crabtree: Pages 159 – 182 and 235 - 266 1 Oxidative Addition Change in Example Group configuration • Oxidative addition is a key step in many transition-metal catalyzed reactions 10 8 I III • Basic reaction: d d Cu Cu 11 X X Pd0 PdII 10 LnM + LnM Pt0 PtII 10 Y Y d8 d6 PdII PdIV 10 • The new M-X and M-Y bonds are formed using the electron pair of the X-Y PtII PtIV 10 bond and one metal-centered lone pair IrI IrIII 9 RhI RhIII 9 • The metal goes up in oxidation state (+2), X-Y formally gets reduced to X-, Y- d6 d4 ReI ReIII 7 • The ease of addition (or elimination) can be tuned by the electronic and 0 II steric properties of the ancillary ligands Mo Mo 6 • OA favored by strongly e-donating L d4 d2 MoII MoIV 6 • The most common applications involve: a) Late transition metals (platinum metals: Ru, Rh, Pd, Ir, Pt) - not d7 d6 2CoII 2CoIII 9 too sensitive to O2 and H2O; routinely used in organic synthesis b) C-Halogen, H-H or Si-H bonds d4 d3 2CrII 2CrIII 6 • Common for transition metals, rare for main-group metals but: Grignard reagents! 2 1 3/1/2021 Oxidative addition – concerted mechanism Concerted addition - mostly with non-polar X-Y bonds X X X LnM + LnM LnM Y Y Y A Cr(CO)5: • H2, silanes, alkanes, ... coordinatively Cr(PMe3)5: • Arene C-H bonds more reactive than alkane C-H bonds unsaturated, but metal- centered lone pairs not phosphines are better • H-H, C-H strong bond, but M-H and M-C bonds can be very available donors, weaker acceptors full oxidative -

Efficient Stille Cross-Coupling Reaction Catalyzed by the Pd(Oac
Efficient Stille Cross-Coupling Reaction found that Dabco was a highly efficient ligand for the - Catalyzed by the Pd(OAc)2/Dabco Catalytic palladium-catalyzed Suzuki Miyaura cross-coupling re- System action resulting in high TONs (up to 950 000 TONs for the reaction of PhI and p-chlorophenylboronic acid) (eq 7,8 Jin-Heng Li,* Yun Liang, De-Ping Wang, Wen-Jie Liu, 1). Thus, we expected to extend the use of this type of Ye-Xiang Xie, and Du-Lin Yin ligand to other palladium-catalyzed transformations. Here, we report a stable, inexpensive, and efficient Key Laboratory of Chemical Biology & Traditional Pd(OAc)2/Dabco catalytic system for the Stille reactions Chinese Medicine Research, College of Chemistry and of aryl halides with organotin compounds. Chemical Engineering, Hunan Normal University, Changsha 410081, China [email protected] Received October 31, 2004 To evaluate the efficiency of Pd(OAc)2/Dabco in the Stille cross-coupling reaction, coupling of 4-bromoanisole (1a) with phenyltributyltin (2a) was first tested, and the An efficient Pd(OAc)2/Dabco-catalyzed Stille cross-coupling reaction procedure has been developed. In the presence of (2) For recent representative papers on phosphine-palladium cata- Pd(OAc)2 and Dabco (triethylenediamine), various aryl lysts, see: (a) Litter, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 1999, 38, halides including aryl iodides, aryl bromides, and activated 2411. (b) Grasa, G. A.; Nolan, S. P. Org. Lett. 2001, 3, 119. (c) Litter, aryl chlorides were coupled efficiently with organotin com- A. F.; Schwarz, L.; Fu, G. C. J. -

Stille and Suzuki-Miyaura Reactions
molecules Article Recoverable Palladium-Catalyzed Carbon-Carbon Bond Forming Reactions under Thermomorphic Mode: Stille and Suzuki-Miyaura Reactions Eskedar Tessema 1,†, Vijayanath Elakkat 1,† , Chiao-Fan Chiu 2,3,*, Zong-Lin Tsai 1, Ka Long Chan 1 , Chia-Rui Shen 4,5, Han-Chang Su 6 and Norman Lu 1,7,* 1 Institute of Organic and Polymeric Materials, National Taipei University of Technology, Taipei 106, Taiwan; [email protected] (E.T.); [email protected] (V.E.); [email protected] (Z.-L.T.); [email protected] (K.L.C.) 2 Department of Pediatrics, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan 3 Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan 4 Department of Medical Biotechnology and Laboratory Sciences, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan; [email protected] 5 Department of Ophthalmology, Linkou Medical Center, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan 6 Creditable Service Technology Consultants, New Taipei City 235, Taiwan; [email protected] 7 Development Center for Smart Textile, National Taipei University of Technology, Taipei 106, Taiwan * Correspondence: [email protected] (C.-F.C.); [email protected] (N.L.). † These authors contributed equally to this work. Citation: Tessema, E.; Elakkat, V.; 0 0 Chiu, C.-F.; Tsai, Z.-L.; Chan, K.L.; Abstract: The reaction of [PdCl2(CH3CN)2] and bis-4,4 -(RfCH2OCH2)-2,2 -bpy (1a–d), where Rf = n- Shen, C.-R.; Su, H.-C.; Lu, N. C11F23 (a), n-C10F21 (b), n-C9F19 (c) and n-C8F17 (d), respectively, in the presence of dichloromethane 0 0 Recoverable Palladium-Catalyzed (CH2Cl2) resulted in the synthesis of Pd complex, [PdCl2[4,4 -bis-(RfCH2OCH2)-2,2 -bpy] (2a–d). -

Misassigned Natural Products and the Role of Chemical Synthesis in Modern Structure Elucidation K
Reviews K. C. Nicolaou and S. A. Snyder Natural Products Synthesis Chasing Molecules That Were Never There: Misassigned Natural Products and the Role of Chemical Synthesis in Modern Structure Elucidation K. C. Nicolaou* and Scott A. Snyder Keywords: azaspiracid-1 · diazonamide A · natural products · revised structures · total synthesis Angewandte Chemie 1012 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim DOI: 10.1002/anie.200460864 Angew. Chem. Int. Ed. 2005, 44, 1012 – 1044 Angewandte Natural Products Synthesis Chemie Over the course of the past half century, the structural elucidation of From the Contents unknown natural products has undergone a tremendous revolution. Before World War II, a chemist would have relied almost exclusively 1. Introduction 1013 on the art of chemical synthesis, primarily in the form of degradation 2. The State of Modern Structure and derivatization reactions, to develop and test structural hypotheses Elucidation 1015 in a process that often took years to complete when grams of material were available. Today, a battery of advanced spectroscopic methods, 3. The Ramifications of Structural such as multidimensional NMR spectroscopy and high-resolution Misassignments 1026 mass spectrometry, not to mention X-ray crystallography, exist for the 4. Misassignment Case Studies 1029 expeditious assignment of structures to highly complex molecules isolated from nature in milligram or sub-milligram quantities. In fact, 5. Summary and Outlook 1037 it could be argued that the characterization of natural products has become a routine task, one which no longer even requires a reaction flask! This Review makes the case that imaginative detective work and chemical synthesis still have important roles to play in the process of solving natures most intriguing molecular puzzles. -
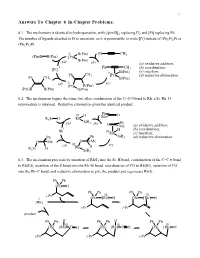
Answers to Chapter 6 In-Chapter Problems
1 Answers To Chapter 6 In-Chapter Problems. 6.1. The mechanism is identical to hydrogenation, with [(pin)B]2 replacing H2 and [Pt] replacing Pd. The number of ligands attached to Pt is uncertain, so it is permissible to write [Pt] instead of (Ph3P)2Pt or (Ph3P)3Pt. II B(Pin) Ph CH3 (Pin)B B(Pin) [Pt] B(Pin) (a) (b) (a) oxidative addition; 0 Ph CH (b) coordination; [Pt] 3 II B(Pin) (c) insertion; Ph CH3 [Pt] (d) reductive elimination. Ph CH3 B(Pin) (d) II (c) [Pt] B(Pin) (Pin)B B(Pin) B(Pin) 6.2. The mechanism begins the same, but after coordination of the C=O π bond to Rh, a Si–Rh–O intermediate is obtained. Reductive elimination gives the identical product. Ph III H Me O R3Si H Rh (a) SiR3 (b) Ph O Me (a) oxidative addition; I H (b) coordination; Rh IIIRh (c) insertion; Ph Ph SiR3 (d) reductive elimination. O Me O Me III (c) (d) Rh H R3Si H SiR3 6.3. The mechanism proceeds by insertion of Rh(I) into the Si–H bond, coordination of the C=C π bond to Rh(III), insertion of the π bond into the Rh–Si bond, coordination of CO to Rh(III), insertion of CO into the Rh–C bond, and reductive elimination to give the product and regenerate Rh(I). Ph Ph OSi H Ph Ph Ph Ph i-Pr III III I OSi [Rh] H OSi [Rh] H [Rh] i-Pr i-Pr product Ph Ph H Ph Ph H Ph Ph III III III OSi [Rh] C O OSi [Rh] C O OSi [Rh] H i-Pr i-Pr i-Pr Chapter 6 2 6.4. -

Role of Electron-Deficient Olefin Ligands in a Ni-Catalyzed Aziridine
pubs.acs.org/JACS Article Role of Electron-Deficient Olefin Ligands in a Ni-Catalyzed Aziridine Cross-Coupling To Generate Quaternary Carbons Jesuś G. Estrada, Wendy L. Williams, Stephen I. Ting, and Abigail G. Doyle* Cite This: J. Am. Chem. Soc. 2020, 142, 8928−8937 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: We previously reported the development of an electron-deficient olefin (EDO) ligand, Fro-DO, that promotes the generation of quaternary carbon centers via Ni-catalyzed Csp3− Csp3 cross-coupling with aziridines. By contrast, electronically and structurally similar EDO ligands such as dimethyl fumarate and electron-deficient styrenes afford primarily β-hydride elimination side reactivity. Only a few catalyst systems have been identified that promote the formation of quaternary carbons via Ni-catalyzed Csp3−Csp3 cross-coupling. Although Fro-DO represents a promis- ing ligand in this regard, the basis for its superior performance is not well understood. Here we describe a detailed mechanistic study of the aziridine cross-coupling reaction and the role of EDO ligands in facilitating Csp3−Csp3 bond formation. This analysis reveals that cross-coupling proceeds by a Ni0/II cycle with a NiII azametallacyclobutane catalyst resting state. Turnover-limiting C−C reductive elimination occurs from a spectroscopically observable NiII-dialkyl intermediate bound to the EDO. Computational analysis shows that Fro-DO accelerates turnover limiting reductive elimination via LUMO lowering. However, it is no more effective than dimethyl fumarate at reducing the barrier to Csp3− Csp3 reductive elimination. Instead, Fro-DO’s unique reactivity arises from its ability to associate favorably to NiII intermediates. -

Regioselectivity Reaction Analytics
Regioselectivity: An Application of Expert Systems and Ontologies to Chemical (Named) Reaction Analysis Roger Sayle, John Mayfield and Noel O’Boyle NextMove Software, Cambridge, UK CINF Reaction Analytics. 256th ACS National Meeting, Boston, MA. Thursday 23rd August 2018 analysis vs. prediction • In “Applied Chemoinformatics” (2018), J. Goodman defines three main problems. – Reaction Planning: R → ? → P [Database] – Reaction Prediction: R1 + R2 → ? [Simulation] – Synthesis Planning: R? + R? + R? → P [Design] • A corollary is that there’s a distinction between reactions that have already been observed and those experiments yet to be performed. CINF Reaction Analytics. 256th ACS National Meeting, Boston, MA. Thursday 23rd August 2018 analysis vs. prediction CINF Reaction Analytics. 256th ACS National Meeting, Boston, MA. Thursday 23rd August 2018 intuition and counter-intuition With apologies to Mark Twain: “Never let the facts get in the way of a good synthesis plan”. There are reactions that chemists expect will happen but don’t & those they don’t expect but do. But cheminformaticians are alchemists that can turn lead into gold, as easily as “[Pb]>>[Au]”. CINF Reaction Analytics. 256th ACS National Meeting, Boston, MA. Thursday 23rd August 2018 Experimental validation • Synthesis of a novel aromatic heterocycle previously unreported in the literature. • William Pitt et al., “Heteroaromatic Rings of the Future”, Journal of Medicinal Chemistry, 52(9):2952- 2963, 2009. CINF Reaction Analytics. 256th ACS National Meeting, Boston, MA. Thursday 23rd August 2018 expectations: setting the scope Goodman Challenges Carey et al. Challenges Maitotoxin Difficult to access substituted aromatic starting materials. Eribulin Org. Biomol. Chem. 2005, 4,2337-2347. CINF Reaction Analytics. -

Hypervalent Iodine Reagents in High Valent Transition Metal Chemistry
molecules Review Hypervalent Iodine Reagents in High Valent Transition Metal Chemistry Felipe Cesar Sousa e Silva, Anthony F. Tierno and Sarah E. Wengryniuk * Department of Chemistry, Temple University, 1901 N. 13th St., Philadelphia, PA 19122, USA; [email protected] (F.C.S.S.); [email protected] (A.F.T.) * Correspondence: [email protected]; Tel.: +1-215-204-0360 Academic Editor: Margaret A. Brimble Received: 29 March 2017; Accepted: 8 May 2017; Published: 12 May 2017 Abstract: Over the last 20 years, high valent metal complexes have evolved from mere curiosities to being at the forefront of modern catalytic method development. This approach has enabled transformations complimentary to those possible via traditional manifolds, most prominently carbon-heteroatom bond formation. Key to the advancement of this chemistry has been the identification of oxidants that are capable of accessing these high oxidation state complexes. The oxidant has to be both powerful enough to achieve the desired oxidation as well as provide heteroatom ligands for transfer to the metal center; these heteroatoms are often subsequently transferred to the substrate via reductive elimination. Herein we will review the central role that hypervalent iodine reagents have played in this aspect, providing an ideal balance of versatile reactivity, heteroatom ligands, and mild reaction conditions. Furthermore, these reagents are environmentally benign, non-toxic, and relatively inexpensive compared to other inorganic oxidants. We will cover advancements in both catalysis and high valent complex isolation with a key focus on the subtle effects that oxidant choice can have on reaction outcome, as well as limitations of current reagents. Keywords: hypervalent iodine; oxidation; oxidant; redox; high valent; high oxidation state; catalysis 1. -

Reversible C–C Bond Formation Using Palladium Catalysis
Reversible C–C Bond Formation Using Palladium Catalysis Austin Marchese University of Toronto https://orcid.org/0000-0002-3090-2748 Bijan Mirabi University of Toronto https://orcid.org/0000-0002-4852-3439 Colton Johnson University of Toronto https://orcid.org/0000-0002-0905-1248 Mark Lautens ( [email protected] ) University of Toronto https://orcid.org/0000-0002-0179-2914 Article Keywords: C-C bonds, reactions, metal-catalyzed reactions Posted Date: May 13th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-426770/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License 1 2 Reversible C–C Bond Formation Using Palladium Catalysis 3 Authors: Austin D. Marchese1†, Bijan Mirabi1†, Colton E. Johnson1, Mark Lautens1* 4 Affiliations: 5 1Department of Chemistry, Davenport Chemical Laboratories, University of Toronto, Toronto, 6 Ontario M5S 3H6, Canada. 7 *Correspondence to: Department of Chemistry, Davenport Chemical Laboratories, University of 8 Toronto, Toronto, Ontario M5S 3H6, Canada; orcid.org/0000-0002-0179- 2914; Email: 9 [email protected]. 10 †These authors contributed equally. 11 Abstract: A widely appreciated principle is that all reactions are fundamentally reversible. 12 Observing reversible transition metal-catalyzed reactions, particularly those that include the 13 cleavage of C–C bonds, are more challenging. The development of the palladium- and nickel- 14 catalyzed carboiodination reactions afforded access to the syn- and anti-diastereomers of the 15 iodo-dihydroisoquinolone products. Using these substrates, an extensive study investigating the 16 catalytic reversibility of the C–C bond formation using a different palladium catalyst was 17 undertaken. -

• Oxidative Addition • Reductive Elimination • Migratory Insertion • Β - Hydrogen Elimination AJELIAS L7-S2 Oxidative Addition
AJELIAS L7-S1 Unique reactions in organometallic chemistry • Oxidative Addition • Reductive Elimination • Migratory Insertion • β - Hydrogen Elimination AJELIAS L7-S2 Oxidative addition When addition of ligands is accompanied by oxidation of the metal, it is called an oxidative addition reaction LnM+XY Ln(X)(Y)M dn dn-2 OX state of metal increases by 2 units H H2 oxidative Ph3P PPh3 addition H PPh3 Coordination number increases by 2 units Rh Rh Ph3P Cl Ph3P Cl PPh3 2 new anionic ligands are added to the metal Rh+1 Rh+3 Requirements for oxidative addition • availability of nonbonded electron density on the metal, • two vacant coordination sites on the reacting complex (LnM), that is, the complex must be coordinatively unsaturated, • a metal with stable oxidation states separated by two units; the higher oxidation state must be energetically accessible and stable. AJELIAS L7-S3 Examples of Oxidative addition : Cis or trans ? Cl Cl PPh3 Ir 18E Cl2 Ph3P CO Cl O Cl PPh3 O2 O PPh3 Ir Ir Ph3P CO Ph3P CO 16E Cl Me Me MeI Cl PPh 3 I PPh3 Ir Ir Ph P CO 3 Ph3P CO I Cl Homonuclear systems (H2, Cl2, O2, C2H2) Cis Heteronuclear systems (MeI) Cis or trans AJELIAS L7-S4 An important step in many homogeneous catalytic cycles Hydrogenation of alkenes- Wilkinson catalyst H H2 oxidative Ph3P PPh3 addition H PPh3 Rh Rh Often thefirststepofmechanism Ph3P Cl Ph3P Cl PPh3 Rh+1 Rh+3 Methanol to acetic acid conversion- Cativa process CH3 I CO CH3I I CO Ir Ir I CO I CO Ir+1 Ir+3 I Pd catalyzed Cross coupling of Ar-B(OH)2 and Ar-X – Suzuki Coupling Br Br Ph3P Pd -

Cross-Coupling Reactions: A
Joesphine Eshon & Prithvi Vangal 1 11th November 2014 . Introduction . Cross-coupling reactions: A. Negishi* Reaction B. Heck* Reaction C. Stille Reaction D. Suzuki* Reaction E. Sonogashira Reaction F. Buchwald-Hartwig Reaction . Summary 2 . Reactions that form (usually) carbon-carbon bonds between complex fragments . Typically use a transition metal catalyst and an organometallic precursor . Most involve a “transmetallation step” . Transmetallation: Transfer of alkyl group from one metal to another . Typical trend: Can transfer from more electropositive to less electropositive metals 3 Pd Catalyst . General reaction scheme: R-X + R . R: Lacks a β hydrogen attached to an sp3 carbon. (Aryl/Benzyl/Vinyl/Allyl) . X: Typically Cl, Br, I, Otf . Regioselectivity and rates are determined by steric hindrance at the alkene CH CHR RCH~ RRC 2 > RRC > HCR > H2C H2C H2C HCR 4 Spessard and Meissler, Organometallic Chemistry . Palladium is in the 0 oxidation state in the active catalyst : PPh3 Pd Ph3P PPh3 e PdCl2 0 . The palladium can be reduced in situ : Pd PPh3 Pd(OAc)2 . Preferred solvent is DMF . Increases rate, lowers temp. to from 800 C : - n Bu N+ ( )4 + KHCO3 . Two catalytic cycles are possible depending on the reaction conditions 5 Spessard and Meissler, Organometallic Chemistry Oxidative Addition: Pd(0) Pd(+2) d10 d8 14 e 16 e 6 Insertion: Pd(+2) Pd(+2) d8 d8 16 e 16 e 7 Hydride elimination: Pd(+2) Pd(+2) d8 d8 16 e 16 e 8 Reductive elimination: Pd(+2) Pd(0) d8 d10 16 e 14 e 9 Example of an intramolecular Heck reaction 10 BINAP . By preventing β elimination from the new sp3 center, an asymmetric product may be formed .