Oxidative Addition & Reductive Elimination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oxidative Addition of Polar Reagents
OXIDATIVE ADDITION OF POLAR REAGENTS Organometallic chemistry has vastly expanded the practicing organic chemist’s notion of what makes a good nucleophile or electrophile. Pre-cross-coupling, for example, using unactivated aryl halides as electrophiles was largely a pipe dream (or possible only under certain specific circumstances). Enter the oxidative addition of polarized bonds: all of a sudden, compounds like bromobenzene started looking a lot more attractive as starting materials. Cross-coupling reactionsinvolving sp2- and sp-hybridized C–X bonds beautifully complement the “classical” substitution reactions at sp3electrophilic carbons. Oxidative addition of the C–X bond is the step that kicks off the magic of these methods. In this post, we’ll explore the mechanisms and favorability trends of oxidative additions of polar reagents. The landscape of mechanistic possibilities for polarized bonds is much more rich than in the non-polar case—concerted, ionic, and radical mechanisms have all been observed. Concerted Mechanisms 2 Oxidative additions of aryl and alkenyl Csp –X bonds, where X is a halogen or sulfonate, proceed through concertedmechanisms analogous to oxidative additions of dihydrogen. Reactions of N–H and O–H bonds in amines, alcohols, and water also appear to be concerted. A π complex involving η2-coordination is an intermediate in the mechanism of insertion into aryl halides at least, and probably vinyl halides too. As two open coordination sites are necessary for concerted oxidative addition, loss of a ligand from a saturated metal complex commonly precedes the actual oxidative addition event. Concerted oxidative addition of aryl halides and sulfonates. Trends in the reactivity of alkyl and aryl (pseudo)halides toward oxidative addition are some of the most famous in organometallic chemistry. -

18- Electron Rule. 18 Electron Rule Cont’D Recall That for MAIN GROUP Elements the Octet Rule Is Used to Predict the Formulae of Covalent Compounds
18- Electron Rule. 18 Electron Rule cont’d Recall that for MAIN GROUP elements the octet rule is used to predict the formulae of covalent compounds. Example 1. This rule assumes that the central atom in a compound will make bonds such Oxidation state of Co? [Co(NH ) ]+3 that the total number of electrons around the central atom is 8. THIS IS THE 3 6 Electron configuration of Co? MAXIMUM CAPACITY OF THE s and p orbitals. Electrons from Ligands? Electrons from Co? This rule is only valid for Total electrons? Period 2 nonmetallic elements. Example 2. Oxidation state of Fe? The 18-electron Rule is based on a similar concept. Electron configuration of Fe? [Fe(CO) ] The central TM can accommodate electrons in the s, p, and d orbitals. 5 Electrons from Ligands? Electrons from Fe? s (2) , p (6) , and d (10) = maximum of 18 Total electrons? This means that a TM can add electrons from Lewis Bases (or ligands) in What can the EAN rule tell us about [Fe(CO)5]? addition to its valence electrons to a total of 18. It can’t occur…… 20-electron complex. This is also known Effective Atomic Number (EAN) Rule Note that it only applies to metals with low oxidation states. 1 2 Sandwich Compounds Obeying EAN EAN Summary 1. Works well only for d-block metals. It does not apply to f-block Let’s draw some structures and see some new ligands. metals. 2. Works best for compounds with TMs of low ox. state. Each of these ligands is π-bonded above and below the metal center. -

NIH Public Access Author Manuscript J Am Chem Soc
NIH Public Access Author Manuscript J Am Chem Soc. Author manuscript; available in PMC 2013 August 29. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Am Chem Soc. 2012 August 29; 134(34): 14232–14237. doi:10.1021/ja306323x. A Mild, Palladium-Catalyzed Method for the Dehydrohalogenation of Alkyl Bromides: Synthetic and Mechanistic Studies Alex C. Bissember†,‡, Anna Levina†, and Gregory C. Fu†,‡ †Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States ‡Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125, United States Abstract We have exploited a typically undesired elementary step in cross-coupling reactions, β-hydride elimination, to accomplish palladium-catalyzed dehydrohalogenations of alkyl bromides to form terminal olefins. We have applied this method, which proceeds in excellent yield at room temperature in the presence of a variety of functional groups, to a formal total synthesis of (R)- mevalonolactone. Our mechanistic studies establish that the rate-determining step can vary with the structure of the alkyl bromide, and, most significantly, that L2PdHBr (L=phosphine), an often- invoked intermediate in palladium-catalyzed processes such as the Heck reaction, is not an intermediate in the active catalytic cycle. INTRODUCTION The elimination of HX to form an olefin is one of the most elementary transformations in organic chemistry (eq 1).1,2 However, harsh conditions, such as the use of a strong Brønsted acid/base or a high temperature (which can lead to poor functional-group compatibility and to olefin isomerization) are often necessary for this seemingly straightforward process. -

Tutorial on Oxidative Addition Jay A
Tutorial pubs.acs.org/Organometallics Tutorial on Oxidative Addition Jay A. Labinger* Beckman Institute and Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 East California Boulevard, Pasadena, California 91125, United States ABSTRACT: This tutorial introduces oxidative addition as a reactivity pattern and organizing principle for organometallic chemistry. The history, characteristics, and scope of oxidative addition are briefly surveyed, followed by a detailed examination of the variety of mechanisms found for the oxidative addition of alkyl halides and their relevance to practical applications. ■ INTRODUCTION comprehensive review but rather as an introduction to the The recognition of oxidative addition as a common pattern of topic, such as might be presented in a lecture, as part of a reactivity has played a central role in the development of course on organometallic chemistry. Accordingly, the tone is organometallic chemistry over the second half of the 20th rather informal, and citations have been limited to a moderate number of historically significant papers. Those interested in century. The starting point for modern organotransition-metal following up on any aspects can find more details and thorough chemistry is usually taken as the discovery and structural referencing elsewhere; Hartwig’s recent textbook5 is a good characterization of ferrocene in the early 1950s (of course, starting point. there were many important earlier contributions). That inspired a large amount of new chemistry during -

Ruthenium Olefin Metathesis Catalysts
Ruthenium Olefin Metathesis Catalysts: Tuning of the Ligand Environment Ruthenium olefine metathese katalysatoren: Optimalisatie van de ligandsfeer Nele Ledoux Promotor: Prof. Dr. F. Verpoort Proefschrift ingediend tot het behalen van de graad van Doctor in de Wetenschappen: Scheikunde Vakgroep Anorganische en Fysische Chemie Vakgroepvoorzitter: Prof. Dr. S. Hoste Faculteit Wetenschappen 2007 ii Members of the Dissertation Committee: Prof. Dr. F. Verpoort Prof. Dr. Ir. C. Stevens Dr. V. Dragutan Dr. R. Drozdzak Dr. R. Winde Prof. Dr. Ir. D. Devos Prof. Dr. J. Van der Eycken Prof. Dr. P. Van Der Voort Prof. Dr. K. Strubbe This research was funded by the Fund for Scien- tific Research - Flanders (F.W.O.-Vlaanderen). Acknowledgments This dissertation is the final product of an educative and fascinating journey, which involved many contributors. First of all, I wish to express my gratitude to the people with whom I learned how to do research. There is my promotor Prof. Dr. Francis Verpoort who gave me the opportunity to join his ’Catalysis group’ and ensured the necessary funding. I’m especially grateful for his confidence and indulgence. In addition, I have been extremely lucky to work with several nice colleagues. Thank you, Thank you (= double thank you ♥) to Bart who helped me conquer many small and big difficulties, and by doing so, contributed a lot to the results reported here. Special thanks to Hans for many funny moments and support over the years. Of course, my acknowledgments also go to the other boys: Carl, Stijn, David, Jeroen, Prof. Dr. Pascal Van Der Voort and not to forget Siegfried, Steven, and Mike for the pleasant working atmosphere and supportive chats. -

Lecture 2--2016
Chem 462 Lecture 2--2016 z [MLnXm] Classification of Ligands Metal Oxidation States Electron Counting Overview of Transition Metal Complexes 1.The coordinate covalent or dative bond applies in L:M 2.Lewis bases are called LIGANDS—all serve as σ-donors some are π-donors as well, and some are π-acceptors 3. Specific coordination number and geometries depend on metal and number of d-electrons 4. HSAB theory useful z [MLnXm] a) Hard bases stabilize high oxidation states b) Soft bases stabilize low oxidation states Classification of Ligands: The L, X, Z Approach Malcolm Green : The CBC Method (or Covalent Bond Classification) used extensively in organometallic chemistry. L ligands are derived from charge-neutral precursors: NH3, amines, N-heterocycles such as pyridine, PR3, CO, alkenes etc. X ligands are derived from anionic precursors: halides, hydroxide, alkoxide alkyls—species that are one-electron neutral ligands, but two electron 4- donors as anionic ligands. EDTA is classified as an L2X4 ligand, features four anions and two neutral donor sites. C5H5 is classified an L2X ligand. Z ligands are RARE. They accept two electrons from the metal center. They donate none. The “ligand” is a Lewis Acid that accepts electrons rather than the Lewis Bases of the X and L ligands that donate electrons. Here, z = charge on the complex unit. z [MLnXm] octahedral Square pyramidal Trigonal bi- pyramidal Tetrahedral Range of Oxidation States in 3d Transition Metals Summary: Classification of Ligands, II: type of donor orbitals involved: σ; σ + π; σ + π*; π+ π* Ligands, Classification I, continued Electron Counting and the famous Sidgewick epiphany Two methods: 1) Neutral ligand (no ligand carries a charge—therefore X ligands are one electron donors, L ligands are 2 electron donors.) 2) Both L and X are 2-electron donor ligand (ionic method) Applying the Ionic or 2-electron donor method: Oxidative addition of H2 to chloro carbonyl bis triphenylphosphine Iridium(I) yields Chloro-dihydrido-carbonyl bis-triphenylphosphine Iridium(III). -

Carbometallation Chemistry
Carbometallation chemistry Edited by Ilan Marek Generated on 01 October 2021, 10:03 Imprint Beilstein Journal of Organic Chemistry www.bjoc.org ISSN 1860-5397 Email: [email protected] The Beilstein Journal of Organic Chemistry is published by the Beilstein-Institut zur Förderung der Chemischen Wissenschaften. This thematic issue, published in the Beilstein Beilstein-Institut zur Förderung der Journal of Organic Chemistry, is copyright the Chemischen Wissenschaften Beilstein-Institut zur Förderung der Chemischen Trakehner Straße 7–9 Wissenschaften. The copyright of the individual 60487 Frankfurt am Main articles in this document is the property of their Germany respective authors, subject to a Creative www.beilstein-institut.de Commons Attribution (CC-BY) license. Carbometallation chemistry Ilan Marek Editorial Open Access Address: Beilstein J. Org. Chem. 2013, 9, 234–235. Schulich Faculty of Chemistry, Technion-Israel Institute of doi:10.3762/bjoc.9.27 Technology, Haifa 32000, Israel Received: 24 January 2013 Email: Accepted: 29 January 2013 Ilan Marek - [email protected] Published: 04 February 2013 This article is part of the Thematic Series "Carbometallation chemistry". Keywords: carbometallation Guest Editor: I. Marek © 2013 Marek; licensee Beilstein-Institut. License and terms: see end of document. Following the pioneering Ziegler addition of nucleophiles to in the 1,2-bisalkylation of nonactivated alkenes! In this nonactivated unsaturated carbon–carbon bonds, the controlled Thematic Series, you will find -

Some Problems in the Oxidative Addition and Binding of an Ethylene to a Transition-Metal Center
Organometallics 1986,5, 1841-1851 1841 Some Problems in the Oxidative Addition and Binding of an Ethylene to a Transition-Metal Center JBr8me Silvestre,laqbMaria Jos6 Calhorda,'a*CRoald Hoffmann, laPage 0. Stoutland,ld and Robert G. Bergmanld Department of Chemistry and MaterriaL Sciences Center, Cornel1 University, Zthaca. New York 14853, Department of Chemistry, University of California, Berkeley, California 94720, and Materials and Molecular Research Division, Lawrence Berkeley Laboratoty, Berkeley, California 94720 Received January 9, 1986 Molecular orbital calculations have been carried out on the potential energy surface of the system CpIrL(C2H4)(L = phosphine). The geometrical and electronic characteristics of the $-olefin and vinyl hydride complexes are examined in some detail. The olefin complex is found to have a slightly lower energy than the vinyl hydride, in agreement with experiment. Different routes are studied which lead to the oxidative addition product from the 16-electron fragment and an ethylene. It is found that an M-H-C linear approach is favored, coupled with a very specific orientation of the ethylenic T system. This results from both electronic and steric requirements. A relatively small barrier is computed for the process,-and the experimentally observed competition between vinyl hydride formation and direct ethylene addition is discussed. Calculations have been performed to ascertain the existence of a separate transition state taking the v2-bound olefin into the vinyl hydride. The computations suggest the existence of two distinct transition states. The influence of substituents and particularly steric effects is analyzed. It is shown how an increase in the size of the ligand affects the shape of the surface and more specifically narrows the energy channels governing conformational changes as well as product formation. -

E Organometallic Rections 1: Reactions at the Metal
E Organometallic Rections 1: Reactions at the Metal Three major classes of reactions: 1 Ligand Substitution . associative (cf. SN2) . dissociative (cf. SN1) . interchange (not dealt with in this course) 2 Oxidative-addition . concerted . step-wise (nucleophilic) . radical 3 Reductive-elimination . concerted cis-elimination . binuclear elimination 1 Ligand Substitution (especially in square planar complexes) Ligand directing effects: some ligands preferentially direct substitution to the site trans to themselves (trans effect). The trans effect of a ligand may be due to: a) destabilization of the trans M-L bond in the ground state (also called the trans influence) Fig. 7-2 text (middle, next page) - - - •strong-donors (H ,PR3,I,Me etc.) weaken the M-L bond trans to themselves • observable by IR (M-L), X-ray (M-L bond length) and NMR (reduced 1 JM-L values) From Organometallic Chemistry by Spessard and Miessler b) stabilization of the transition state (true trans effect) Fig 7-2 text (right) + . strong -acceptors (eg.CO,C2H4,NO etc.) remove electron density in the equatorial plane of 5-coordinate tbp transition states thus decreasing electrostatic repulsion Combining - and -effects gives the observed trans effect order: - - - - - - - - - - CO, CN ,C2H4 > PR3,H > Me > Ph >NO2 ,I,SCN >Br >Cl >py,NH3,OH,H2O + - Eg. trans-Pt(PEt3)2Cl(L) + py trans-Pt(PEt3)2(py)(L) + Cl Lk(M-1s-1) - H , PEt3 4 Ph- 10-2 Cl- 10-4 Associative (A) substitution . most common for sq. planar 16 e- species . usually proceeds with retention of configuration . rate law is 1st order in complex and incoming ligand Y NB: T is a stronger trans labilizing ligand than A or X . -

Roles of Base in the Pd-Catalyzed Annulative Chlorophenylene Dimerization
!"#$%&"'&()%$&*+&,-$&./01),)#23$/&4++5#),*6$& 1-#"7"8-$+2#$+$&9*:$7*3),*"+& Li-Ping Xu,a,b Brandon E. Haines,c Manjaly J. Ajitha,a Kei Murakami,d Kenichiro Itami,*,d and Djamaladdin G. Musaev*,a a Cherry L. Emerson Center for Scientific Computation, Emory University, Atlanta, Georgia 30322, USA b School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, 255000, China c Westmont College, Department of Chemistry, Santa Barbara CA, 93108, USA d Institute of Transformative Bio-Molecules (WPI-ITbM) and Graduate School of Science, and JST-ERATO, Itami Molecular Nanocarbon Project, Nagoya University, Chikusa, Nagoya 464- 8602, Japan KEYWORDS: Palladium catalyst, polycyclic aromatic hydrocarbon, C-H functionalization, DFT, base effect ABSTRACT By using computation, the detailed mechanism of the Pd-catalyzed annulative chlorophenylene dimerization has been elucidated and the roles of the base have been identified. It is shown that the initial steps of this reaction are the active catalyst formation and the first C–Cl bond activation proceeds via the “base-assisted oxidative addition” mechanism. Overall, these steps of the reaction proceed via 16.2 kcal/mol free energy barrier for the C–Cl bond activation and are highly exergonic. Importantly, the base directly participates in the C–Cl bond cleavage introduces a mechanistic switch of the Pd(0)/Pd(II) oxidation and makes the reaction more exergonic. The following steps of the reaction are palladacycle and Pd-aryne formations, among which the palladacycle formation is found to be favorable: it proceeds with a moderate C–H activation barrier and is slightly exergonic. Although the Pd-aryne formation requires a slightly lower energy barrier, it is highly endergonic: therefore, the participation of the Pd-aryne complex, or its derivatives, in the Pd- catalyzed annulative chlorophenylene dimerization in the presence of Cs2CO3 seems to be unlikely. -
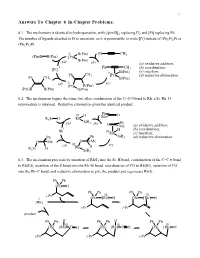
Answers to Chapter 6 In-Chapter Problems
1 Answers To Chapter 6 In-Chapter Problems. 6.1. The mechanism is identical to hydrogenation, with [(pin)B]2 replacing H2 and [Pt] replacing Pd. The number of ligands attached to Pt is uncertain, so it is permissible to write [Pt] instead of (Ph3P)2Pt or (Ph3P)3Pt. II B(Pin) Ph CH3 (Pin)B B(Pin) [Pt] B(Pin) (a) (b) (a) oxidative addition; 0 Ph CH (b) coordination; [Pt] 3 II B(Pin) (c) insertion; Ph CH3 [Pt] (d) reductive elimination. Ph CH3 B(Pin) (d) II (c) [Pt] B(Pin) (Pin)B B(Pin) B(Pin) 6.2. The mechanism begins the same, but after coordination of the C=O π bond to Rh, a Si–Rh–O intermediate is obtained. Reductive elimination gives the identical product. Ph III H Me O R3Si H Rh (a) SiR3 (b) Ph O Me (a) oxidative addition; I H (b) coordination; Rh IIIRh (c) insertion; Ph Ph SiR3 (d) reductive elimination. O Me O Me III (c) (d) Rh H R3Si H SiR3 6.3. The mechanism proceeds by insertion of Rh(I) into the Si–H bond, coordination of the C=C π bond to Rh(III), insertion of the π bond into the Rh–Si bond, coordination of CO to Rh(III), insertion of CO into the Rh–C bond, and reductive elimination to give the product and regenerate Rh(I). Ph Ph OSi H Ph Ph Ph Ph i-Pr III III I OSi [Rh] H OSi [Rh] H [Rh] i-Pr i-Pr product Ph Ph H Ph Ph H Ph Ph III III III OSi [Rh] C O OSi [Rh] C O OSi [Rh] H i-Pr i-Pr i-Pr Chapter 6 2 6.4. -

Role of Electron-Deficient Olefin Ligands in a Ni-Catalyzed Aziridine
pubs.acs.org/JACS Article Role of Electron-Deficient Olefin Ligands in a Ni-Catalyzed Aziridine Cross-Coupling To Generate Quaternary Carbons Jesuś G. Estrada, Wendy L. Williams, Stephen I. Ting, and Abigail G. Doyle* Cite This: J. Am. Chem. Soc. 2020, 142, 8928−8937 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: We previously reported the development of an electron-deficient olefin (EDO) ligand, Fro-DO, that promotes the generation of quaternary carbon centers via Ni-catalyzed Csp3− Csp3 cross-coupling with aziridines. By contrast, electronically and structurally similar EDO ligands such as dimethyl fumarate and electron-deficient styrenes afford primarily β-hydride elimination side reactivity. Only a few catalyst systems have been identified that promote the formation of quaternary carbons via Ni-catalyzed Csp3−Csp3 cross-coupling. Although Fro-DO represents a promis- ing ligand in this regard, the basis for its superior performance is not well understood. Here we describe a detailed mechanistic study of the aziridine cross-coupling reaction and the role of EDO ligands in facilitating Csp3−Csp3 bond formation. This analysis reveals that cross-coupling proceeds by a Ni0/II cycle with a NiII azametallacyclobutane catalyst resting state. Turnover-limiting C−C reductive elimination occurs from a spectroscopically observable NiII-dialkyl intermediate bound to the EDO. Computational analysis shows that Fro-DO accelerates turnover limiting reductive elimination via LUMO lowering. However, it is no more effective than dimethyl fumarate at reducing the barrier to Csp3− Csp3 reductive elimination. Instead, Fro-DO’s unique reactivity arises from its ability to associate favorably to NiII intermediates.