Unique Features Revealed by the Genome Sequence of Acinetobacter Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. Significant
Table 2. Significant (Q < 0.05 and |d | > 0.5) transcripts from the meta-analysis Gene Chr Mb Gene Name Affy ProbeSet cDNA_IDs d HAP/LAP d HAP/LAP d d IS Average d Ztest P values Q-value Symbol ID (study #5) 1 2 STS B2m 2 122 beta-2 microglobulin 1452428_a_at AI848245 1.75334941 4 3.2 4 3.2316485 1.07398E-09 5.69E-08 Man2b1 8 84.4 mannosidase 2, alpha B1 1416340_a_at H4049B01 3.75722111 3.87309653 2.1 1.6 2.84852656 5.32443E-07 1.58E-05 1110032A03Rik 9 50.9 RIKEN cDNA 1110032A03 gene 1417211_a_at H4035E05 4 1.66015788 4 1.7 2.82772795 2.94266E-05 0.000527 NA 9 48.5 --- 1456111_at 3.43701477 1.85785922 4 2 2.8237185 9.97969E-08 3.48E-06 Scn4b 9 45.3 Sodium channel, type IV, beta 1434008_at AI844796 3.79536664 1.63774235 3.3 2.3 2.75319499 1.48057E-08 6.21E-07 polypeptide Gadd45gip1 8 84.1 RIKEN cDNA 2310040G17 gene 1417619_at 4 3.38875643 1.4 2 2.69163229 8.84279E-06 0.0001904 BC056474 15 12.1 Mus musculus cDNA clone 1424117_at H3030A06 3.95752801 2.42838452 1.9 2.2 2.62132809 1.3344E-08 5.66E-07 MGC:67360 IMAGE:6823629, complete cds NA 4 153 guanine nucleotide binding protein, 1454696_at -3.46081884 -4 -1.3 -1.6 -2.6026947 8.58458E-05 0.0012617 beta 1 Gnb1 4 153 guanine nucleotide binding protein, 1417432_a_at H3094D02 -3.13334396 -4 -1.6 -1.7 -2.5946297 1.04542E-05 0.0002202 beta 1 Gadd45gip1 8 84.1 RAD23a homolog (S. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Transcriptomic and Proteomic Profiling Provides Insight Into
BASIC RESEARCH www.jasn.org Transcriptomic and Proteomic Profiling Provides Insight into Mesangial Cell Function in IgA Nephropathy † † ‡ Peidi Liu,* Emelie Lassén,* Viji Nair, Celine C. Berthier, Miyuki Suguro, Carina Sihlbom,§ † | † Matthias Kretzler, Christer Betsholtz, ¶ Börje Haraldsson,* Wenjun Ju, Kerstin Ebefors,* and Jenny Nyström* *Department of Physiology, Institute of Neuroscience and Physiology, §Proteomics Core Facility at University of Gothenburg, University of Gothenburg, Gothenburg, Sweden; †Division of Nephrology, Department of Internal Medicine and Department of Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, Michigan; ‡Division of Molecular Medicine, Aichi Cancer Center Research Institute, Nagoya, Japan; |Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden; and ¶Integrated Cardio Metabolic Centre, Karolinska Institutet Novum, Huddinge, Sweden ABSTRACT IgA nephropathy (IgAN), the most common GN worldwide, is characterized by circulating galactose-deficient IgA (gd-IgA) that forms immune complexes. The immune complexes are deposited in the glomerular mesangium, leading to inflammation and loss of renal function, but the complete pathophysiology of the disease is not understood. Using an integrated global transcriptomic and proteomic profiling approach, we investigated the role of the mesangium in the onset and progression of IgAN. Global gene expression was investigated by microarray analysis of the glomerular compartment of renal biopsy specimens from patients with IgAN (n=19) and controls (n=22). Using curated glomerular cell type–specific genes from the published literature, we found differential expression of a much higher percentage of mesangial cell–positive standard genes than podocyte-positive standard genes in IgAN. Principal coordinate analysis of expression data revealed clear separation of patient and control samples on the basis of mesangial but not podocyte cell–positive standard genes. -
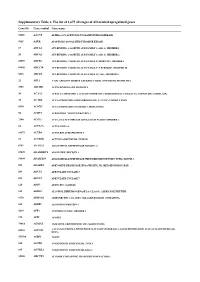
Supplementary Table 1. the List of 1,675 All Stages of AD-Related Upregulated Genes
Supplementary Table 1. The list of 1,675 all stages of AD-related upregulated genes Gene ID Gene symbol Gene name 51146 A4GNT ALPHA-1,4-N-ACETYLGLUCOSAMINYLTRANSFERASE 9625 AATK APOPTOSIS-ASSOCIATED TYROSINE KINASE 19 ABCA1 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 1 20 ABCA2 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 2 10058 ABCB6 ATP-BINDING CASSETTE, SUB-FAMILY B (MDR/TAP), MEMBER 6 89845 ABCC10 ATP-BINDING CASSETTE, SUB-FAMILY C (CFTR/MRP), MEMBER 10 5826 ABCD4 ATP-BINDING CASSETTE, SUB-FAMILY D (ALD), MEMBER 4 25 ABL1 V-ABL ABELSON MURINE LEUKEMIA VIRAL ONCOGENE HOMOLOG 1 3983 ABLIM1 ACTIN BINDING LIM PROTEIN 1 30 ACAA1 ACETYL-COENZYME A ACYLTRANSFERASE 1 (PEROXISOMAL 3-OXOACYL-COENZYME A THIOLASE) 35 ACADS ACYL-COENZYME A DEHYDROGENASE, C-2 TO C-3 SHORT CHAIN 8310 ACOX3 ACYL-COENZYME A OXIDASE 3, PRISTANOYL 56 ACRV1 ACROSOMAL VESICLE PROTEIN 1 2180 ACSL1 ACYL-COA SYNTHETASE LONG-CHAIN FAMILY MEMBER 1 86 ACTL6A ACTIN-LIKE 6A 93973 ACTR8 ACTIN-RELATED PROTEIN 8 91 ACVR1B ACTIVIN A RECEPTOR, TYPE IB 8747 ADAM21 ADAM METALLOPEPTIDASE DOMAIN 21 27299 ADAMDEC1 ADAM-LIKE, DECYSIN 1 56999 ADAMTS9 ADAM METALLOPEPTIDASE WITH THROMBOSPONDIN TYPE 1 MOTIF, 9 105 ADARB2 ADENOSINE DEAMINASE, RNA-SPECIFIC, B2 (RED2 HOMOLOG RAT) 109 ADCY3 ADENYLATE CYCLASE 3 113 ADCY7 ADENYLATE CYCLASE 7 120 ADD3 ADDUCIN 3 (GAMMA) 125 ADH1C ALCOHOL DEHYDROGENASE 1A (CLASS I), ALPHA POLYPEPTIDE 9370 ADIPOQ ADIPONECTIN, C1Q AND COLLAGEN DOMAIN CONTAINING 165 AEBP1 AE BINDING PROTEIN 1 4299 AFF1 AF4/FMR2 FAMILY, MEMBER 1 173 AFM AFAMIN 79814 AGMAT AGMATINE -

Enzymes Important in the KREBS-HENSELEIT UREA SYNTHESIZING CYCLE* ARGINASE ORNITHINE CARBAMOYLTRANSFERASE (E.C. 3.5,3,1) from Beef Liver (E.C
enzymes important in the KREBS-HENSELEIT UREA SYNTHESIZING CYCLE* ARGINASE ORNITHINE CARBAMOYLTRANSFERASE (E.C. 3.5,3,1) from Beef Liver (E.C. 2.1.3.3) from Streptococcus faecalis (L-Arginine Ureohydrolase; (Ornithine Transcarbamylase; Carbamoylphosphate: L-Arginine amidinohydrolase) L-Ornithine Carbamoyltransferase) Lyophilized powder Lyophilized with Trizma ® buffer. Catalyzes the following reaction: SuitableforthedeterminationofCarbamyl Phosphate L-Arginine + H~O-*L-Ornithine + Urea and Ornithine, One unit will convert approximately one micromole One unit will form one micromole of Citrulline from of L(+) Arginine to L-Ornithine and Urea per minute Ornithine and Carbamyl Phosphate I~er minute at at pH 9.5 at 37°C. pH 8.5 at 37°C. Order Sigma Product No. A-2137 Order Sigma Product No. O-2501 Approx. 60 units per mg. Protein (Biuret) Approx. 600 units per mg. Protein (Lowry) 2500 units $ 4.85 12,500 units $20.00 30 units $5.00 300 units $40.00 6250 units 11.00 25,000 units 30.00 *Ref.: Krebs, H. A. and Henseleit, K., Z. Physiol. Chem., 210:33 ().932). Other enzymes related to AMMONIA NITROGEN METABOLISM: L-Asparaginase Glutamic Oxalacetic Transaminase Glutaminase L-Aspartase Glutamic-Pyruvic Transaminase L-Histidase Creatininase Gabase Adenosine Deaminase L-Glutamic Dehydrogenase Useful for assay of'r-Aminobutyric Acid. 5'-AMP Deaminase See the Sigma Catalog for further details. ~//~"~ CARBOXYPEPTIDASE - B (E. C. No. 3.4.2.2.) catalyzes hydrolysis of the basic amino acids Arginine and Lysine from the carboxyl terminal position in polypeptides. One of the major pancreatic proteases, Carboxypeptidase-B also functions in the further degradation of products of tryptic digestion. -

Structure and Function of Metallohydrolases in the Arginase- Deacetylase Family
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2016 Structure and Function of Metallohydrolases in the Arginase- Deacetylase Family Yang Hai University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Biochemistry Commons Recommended Citation Hai, Yang, "Structure and Function of Metallohydrolases in the Arginase-Deacetylase Family" (2016). Publicly Accessible Penn Dissertations. 1753. https://repository.upenn.edu/edissertations/1753 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/1753 For more information, please contact [email protected]. Structure and Function of Metallohydrolases in the Arginase-Deacetylase Family Abstract Arginases and deacetylases are metallohydrolases that catalyze two distinct chemical transformations. The arginases catalyze the hydrolysis of the guanidinium group of arginine by using a hydroxide ion 2+ 2+ bridging the binuclear manganese cluster (Mn A-Mn B) for nucleophilic attack. The deacetylases catalyze the hydrolysis of amide bonds by using a mononuclear Zn2+-ion activated water molecule as the nucleophile. Despite the diverse functions, metallohydrolases of the arginase-deacetylase superfamily 2+ share the same characteristic α/β hydrolase core fold and a conserved metal binding site (the Mn B site in arginase corresponds to the catalytic Zn2+ site in deacetylase) which is essential for catalysis in both enzymes. We report crystal structure of formiminoglutamase from the parasitic protozoan Trypanosoma cruzi and confirm that formiminoglutamase is a Mn2+-requiring hydrolase that belongs to the arginase- deacetylase superfamily. We also report the crystal structure of an arginase-like protein from Trypanosoma brucei (TbARG) with unknown function. Although its biological role remains enigmatic, the 2+ evolutionarily more conserved Mn B site can be readily restored in TbARG through side-directed mutagenesis. -

Inositol Phosphate in the Basidiomycete Fungus Schizophyllum Commune
Inositol phosphate in the basidiomycete fungus Schizophyllum commune Dissertation To fulfill the Requirements for the Degree of „doctor rerum naturalium“ (Dr. rer. nat.) Submitted to the Council of the Faculty of Biological Science of the Friedrich Schiller University Jena by Reyna Carmina Felicia Murry, MSc. born on the 22nd of October 1987 in Bandung, Indonesia First reviewer: Prof. Dr. Erika Kothe, Institut für Mikrobiologie, FSU Jena Second Reviewer: Prof. Dr. Axel A. Brakhage, Institut für Mikrobiologie, FSU Jena Third Reviewer: Prof. Dr. J. Stephen Horton, Department of Biological Sciences, Science and Engineering Center, Union College, Schenectady, New York, USA Date of public defense: 7th May 2019 “Science is the key to our future, and if you don't believe in science, then you're holding everybody back.” Bill Nye Table of Contents 1 Introduction ......................................................................................................................... 1 1.1 Schizophyllum commune .............................................................................................. 1 1.2 Fruiting body development in S. commune ................................................................. 3 1.3 Signal transduction: G-protein-coupled receptors (GPCRs) and Ras signaling .......... 4 1.4 Inositol-based metabolism and signaling: inositol phosphates and inositol lipids ...... 5 1.5 Inositol and inositol monophosphatase ........................................................................ 7 1.6 Inositol hexakisphosphate and -

Generated by SRI International Pathway Tools Version 25.0, Authors S
An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_001836235-HmpCyc: Bacillus sp. HMSC036E02 Cellular Overview Connections between pathways are omitted for legibility. -

Comprehensive Proteomic Study of Bacillus Amyloliquefaciens Strain FZB42 and Its Response to Plant Root Exudates
Comprehensive proteomic study of Bacillus amyloliquefaciens strain FZB42 and its response to plant root exudates Dissertation zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) im Fach Biologie eingereicht an der Mathematisch-Naturwissenschaftlichen Fakultät I der Humboldt-Univesität zu Berlin von M. Sc. Kinga Kierul, Geburtsname Szczypinska Präsident der Humboldt-Universität zu Berlin Prof. Dr. Jan-Hendrik Olbertz Dekan der Mathematisch-Naturwissenschaftlichen Fakultät I Prof. Dr. Andreas Herrmann Gutachter: 1. Prof. Dr. Rainer Borriss 2. Prof. Dr. Thomas Schweder 3. Prof. Dr. Thomas Eitinger Tag der mündlichen Prüfung: 21.08.12 SUMMARY ...................................................................................................................................IX 1 INTRODUCTION ................................................................................................................... 1 1.1 PLANT-MICROBE INTERACTIONS ............................................................................................... 1 1.2 PLANT-ROOT COLONIZATION .................................................................................................... 2 1.3 PLANT-DERIVED COMPOUNDS IN THE SOIL AND THEIR FUNCTION ........................................... 2 1.4 MECHANISMS OF EXUDATION ................................................................................................... 3 1.5 PLANT ASSOCIATED BACTERIA AND THEIR USE IN AGRICULTURE ............................................ 4 1.6 WHAT MAKES B. AMYLOLIQUEFACIENS -

Supplemental Figures 04 12 2017
Jung et al. 1 SUPPLEMENTAL FIGURES 2 3 Supplemental Figure 1. Clinical relevance of natural product methyltransferases (NPMTs) in brain disorders. (A) 4 Table summarizing characteristics of 11 NPMTs using data derived from the TCGA GBM and Rembrandt datasets for 5 relative expression levels and survival. In addition, published studies of the 11 NPMTs are summarized. (B) The 1 Jung et al. 6 expression levels of 10 NPMTs in glioblastoma versus non‐tumor brain are displayed in a heatmap, ranked by 7 significance and expression levels. *, p<0.05; **, p<0.01; ***, p<0.001. 8 2 Jung et al. 9 10 Supplemental Figure 2. Anatomical distribution of methyltransferase and metabolic signatures within 11 glioblastomas. The Ivy GAP dataset was downloaded and interrogated by histological structure for NNMT, NAMPT, 12 DNMT mRNA expression and selected gene expression signatures. The results are displayed on a heatmap. The 13 sample size of each histological region as indicated on the figure. 14 3 Jung et al. 15 16 Supplemental Figure 3. Altered expression of nicotinamide and nicotinate metabolism‐related enzymes in 17 glioblastoma. (A) Heatmap (fold change of expression) of whole 25 enzymes in the KEGG nicotinate and 18 nicotinamide metabolism gene set were analyzed in indicated glioblastoma expression datasets with Oncomine. 4 Jung et al. 19 Color bar intensity indicates percentile of fold change in glioblastoma relative to normal brain. (B) Nicotinamide and 20 nicotinate and methionine salvage pathways are displayed with the relative expression levels in glioblastoma 21 specimens in the TCGA GBM dataset indicated. 22 5 Jung et al. 23 24 Supplementary Figure 4. -

Fungal and Host Transcriptome Analysis of Ph-Regulated Genes
Barad et al. BMC Genomics (2016) 17:330 DOI 10.1186/s12864-016-2665-7 RESEARCH ARTICLE Open Access Fungal and host transcriptome analysis of pH-regulated genes during colonization of apple fruits by Penicillium expansum Shiri Barad1,2†,NoaSela3†, Dilip Kumar1, Amit Kumar-Dubey1, Nofar Glam-Matana1,2,AmirSherman4 and Dov Prusky1* Abstract Background: Penicillium expansum is a destructive phytopathogen that causes decay in deciduous fruits during postharvest handling and storage. During colonization the fungus secretes D-gluconic acid (GLA), which modulates environmental pH and regulates mycotoxin accumulation in colonized tissue. Till now no transcriptomic analysis has addressed the specific contribution of the pathogen's pH regulation to the P. expansum colonization process. For this purpose total RNA from the leading edge of P. expansum-colonized apple tissue of cv. 'Golden Delicious' and from fungal cultures grown under pH 4 or 7 were sequenced and their gene expression patterns were compared. Results: We present a large-scale analysis of the transcriptome data of P. expansum and apple response to fungal colonization. The fungal analysis revealed nine different clusters of gene expression patterns that were divided among three major groups in which the colonized tissue showed, respectively: (i) differing transcript expression patterns between mycelial growth at pH 4 and pH 7; (ii) similar transcript expression patterns of mycelial growth at pH 4; and (iii) similar transcript expression patterns of mycelial growth at pH 7. Each group was functionally characterized in order to decipher genes that are important for pH regulation and also for colonization of apple fruits by Penicillium. Furthermore, comparison of gene expression of healthy apple tissue with that of colonized tissue showed that differentially expressed genes revealed up-regulation of the jasmonic acid and mevalonate pathways, and also down-regulation of the glycogen and starch biosynthesis pathways. -

Supplementary Figure 1 a C E G B D
Supplementary Figure 1 A APC B TP53 50 50 N/S N/S 40 40 30 30 20 20 10 10 Doubling time (h), SRB (h), Doublingtime Doubling time (h), SRB Doubling time n= 830n= n= 16n= 26 0 0 wt mut wt mut KRAS BRAF C 50 D 50 N/S 40 40 N/S 30 30 20 20 10 10 Doubling time (h), SRB Doubling time (h), SRB (h), time Doubling n= 19n= 29 n= 39n= 7 0 0 wt mut wt mut SMAD4 E F 60 N/S 40 20 27 12 (h), SRB time Doubling n= 20n= 5 0 wt mut G TCF7L2 H CTNNB1 60 50 N/S N/S 40 40 30 20 20 10 Doubling time (h),SRB n= 19n= 6 Doubling (h), SRB time n= 19n= 6 0 0 wt mut wt mut Supplementary Figure 1: Cell growth as a function of the mutational status of the most frequently mutated genes in colorectal tumors. The average doubling time of cell lines that are either wild type or mutant for APC (A), TP53 (B), KRAS (C), BRAF (D), PIK3CA (E), SMAD4 (F), TCF7L2 (G)andCTNNB1 (H) was calculated to assess the possible effects on tumor growth of the most frequent mutations observed in colorectal tumors. N/S: Student’s T‐test p>0.05. Supplementary Figure 2 A B 20000 GAPDH TYMS 4000 15000 3000 10000 2000 5000 1554696_s_at 1000 Pearson r =0.459 Pearson r =0.457 P value =0.024 AFFX-HUMGAPDH/M33197_5_at 0 P value =0.037 Relative expression (microarray) 0 0 20 40 60 80 100 Relative expression (microarray) expression Relative 0 20 40 60 80 100 Relative expression (qPCR) Relative expression (qPCR) C D PPOX CALCOCO2 80 80 60 60 40 40 238117_at 238560_at 20 20 Pearson r =0.590 Pearson r =0.772 P value =0.004 P value =0.025 0 0 Relative expression (microarray) 0 20 40 60 80 100 (microarray) Relative expression 0 100 200 300 400 500 Relative expression (qPCR) Relative expression (qPCR) E F CBX5 SMAD4 400 300 300 200 200 231862_at 202526_at 100 100 Pearson r =0.873 Pearson r =0.898 P value =0.015 0 P value =0.001 0 Relative expression (microarray) 0 100 200 300 400 500 Relative expression (microarray) 0 100 200 300 Relative expression (qPCR) Relative expression (qPCR) Supplementary Figure 2: Independent validation of mRNA expression levels.