University of Birmingham the Histone-Deacetylase-Inhibitor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. Significant
Table 2. Significant (Q < 0.05 and |d | > 0.5) transcripts from the meta-analysis Gene Chr Mb Gene Name Affy ProbeSet cDNA_IDs d HAP/LAP d HAP/LAP d d IS Average d Ztest P values Q-value Symbol ID (study #5) 1 2 STS B2m 2 122 beta-2 microglobulin 1452428_a_at AI848245 1.75334941 4 3.2 4 3.2316485 1.07398E-09 5.69E-08 Man2b1 8 84.4 mannosidase 2, alpha B1 1416340_a_at H4049B01 3.75722111 3.87309653 2.1 1.6 2.84852656 5.32443E-07 1.58E-05 1110032A03Rik 9 50.9 RIKEN cDNA 1110032A03 gene 1417211_a_at H4035E05 4 1.66015788 4 1.7 2.82772795 2.94266E-05 0.000527 NA 9 48.5 --- 1456111_at 3.43701477 1.85785922 4 2 2.8237185 9.97969E-08 3.48E-06 Scn4b 9 45.3 Sodium channel, type IV, beta 1434008_at AI844796 3.79536664 1.63774235 3.3 2.3 2.75319499 1.48057E-08 6.21E-07 polypeptide Gadd45gip1 8 84.1 RIKEN cDNA 2310040G17 gene 1417619_at 4 3.38875643 1.4 2 2.69163229 8.84279E-06 0.0001904 BC056474 15 12.1 Mus musculus cDNA clone 1424117_at H3030A06 3.95752801 2.42838452 1.9 2.2 2.62132809 1.3344E-08 5.66E-07 MGC:67360 IMAGE:6823629, complete cds NA 4 153 guanine nucleotide binding protein, 1454696_at -3.46081884 -4 -1.3 -1.6 -2.6026947 8.58458E-05 0.0012617 beta 1 Gnb1 4 153 guanine nucleotide binding protein, 1417432_a_at H3094D02 -3.13334396 -4 -1.6 -1.7 -2.5946297 1.04542E-05 0.0002202 beta 1 Gadd45gip1 8 84.1 RAD23a homolog (S. -

Effect of a Caloric Restriction Based On
UNIVERSIDAD AUTÓNOMA DE MADRID FACULTAD DE CIENCIAS DEPARTAMENTO DE BIOLOGÍA DOCTORAL THESIS Biology PhD “Effect of a caloric restriction based on the Mediterranean diet and intake of traditional Mediterranean foods on the expression of microRNAs regulating molecular processes associated with aging” INSTITUTO MADRILEÑO DE ESTUDIOS AVANZADOS EN ALIMENTACIÓN (IMDEA FOOD INSTITUTE) VÍCTOR MICÓ MORENO Madrid, 2018 UNIVERSIDAD AUTÓNOMA DE MADRID FACULTAD DE CIENCIAS DEPARTAMENTO DE BIOLOGÍA DOCTORAL THESIS Biology PhD “Effect of a caloric restriction based on the Mediterranean diet and intake of traditional Mediterranean foods on the expression of microRNAs regulating molecular processes associated with aging” INSTITUTO MADRILEÑO DE ESTUDIOS AVANZADOS EN ALIMENTACIÓN (IMDEA FOOD INSTITUTE) Memoria presentada por: Víctor Micó Moreno Para optar al grado de: DOCTOR EN BIOLOGÍA Doña Lidia Ángeles Daimiel Ruíz, Doctora en Biología Celular y Genética por la Universidad Autónoma de Madrid, investigadora del Instituto IMDEA Alimentación, informa favorablemente la solicitud de autorización de defensa de la tesis doctoral con el Título: “Effect of a caloric restriction based on the Mediterranean diet and intake of traditional Mediterranean foods on the expression of microRNAs regulating molecular processes associated with aging”, presentada por Don Víctor Micó Moreno para optar al grado de Doctor en Biología. Este trabajo ha sido realizado en el Instituto Madrileño de Estudios Avanzados en Alimentación (IMDEA Alimentación) bajo su dirección, y cumple satisfactoriamente las condiciones requeridas por el Departamento de Biología de la Universidad Autónoma de Madrid para optar al Título de Doctor. Ha actuado como tutor académico, y presenta su conformidad el Dr. Carlos Francisco Sentís Castaño, vicedecano de Personal Docente e Investigador y profesor titular del Departamento de Biología de la Facultad de Ciencias de la Universidad Autónoma de Madrid. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Transcriptomic and Proteomic Profiling Provides Insight Into
BASIC RESEARCH www.jasn.org Transcriptomic and Proteomic Profiling Provides Insight into Mesangial Cell Function in IgA Nephropathy † † ‡ Peidi Liu,* Emelie Lassén,* Viji Nair, Celine C. Berthier, Miyuki Suguro, Carina Sihlbom,§ † | † Matthias Kretzler, Christer Betsholtz, ¶ Börje Haraldsson,* Wenjun Ju, Kerstin Ebefors,* and Jenny Nyström* *Department of Physiology, Institute of Neuroscience and Physiology, §Proteomics Core Facility at University of Gothenburg, University of Gothenburg, Gothenburg, Sweden; †Division of Nephrology, Department of Internal Medicine and Department of Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, Michigan; ‡Division of Molecular Medicine, Aichi Cancer Center Research Institute, Nagoya, Japan; |Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden; and ¶Integrated Cardio Metabolic Centre, Karolinska Institutet Novum, Huddinge, Sweden ABSTRACT IgA nephropathy (IgAN), the most common GN worldwide, is characterized by circulating galactose-deficient IgA (gd-IgA) that forms immune complexes. The immune complexes are deposited in the glomerular mesangium, leading to inflammation and loss of renal function, but the complete pathophysiology of the disease is not understood. Using an integrated global transcriptomic and proteomic profiling approach, we investigated the role of the mesangium in the onset and progression of IgAN. Global gene expression was investigated by microarray analysis of the glomerular compartment of renal biopsy specimens from patients with IgAN (n=19) and controls (n=22). Using curated glomerular cell type–specific genes from the published literature, we found differential expression of a much higher percentage of mesangial cell–positive standard genes than podocyte-positive standard genes in IgAN. Principal coordinate analysis of expression data revealed clear separation of patient and control samples on the basis of mesangial but not podocyte cell–positive standard genes. -

Compartmentation of NAD+-Dependent Signalling ⇑ ⇑ Friedrich Koch-Nolte A, , Stefan Fischer B, Friedrich Haag A, Mathias Ziegler B
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector FEBS Letters 585 (2011) 1651–1656 journal homepage: www.FEBSLetters.org Review Compartmentation of NAD+-dependent signalling ⇑ ⇑ Friedrich Koch-Nolte a, , Stefan Fischer b, Friedrich Haag a, Mathias Ziegler b, a Institute of Immunology, University Medical Center, Martinistr. 52, 20246 Hamburg, Germany b Department of Molecular Biology, University of Bergen, Thormøhlensgate 55, 5008 Bergen, Norway article info abstract Article history: NAD+ plays central roles in energy metabolism as redox carrier. Recent research has identified Received 25 February 2011 important signalling functions of NAD+ that involve its consumption. Although NAD+ is synthesized Revised 21 March 2011 mainly in the cytosol, nucleus and mitochondria, it has been detected also in vesicular and extracel- Accepted 21 March 2011 lular compartments. Three protein families that consume NAD+ in signalling reactions have been Available online 31 March 2011 characterized on a molecular level: ADP-ribosyltransferases (ARTs), Sirtuins (SIRTs), and NAD+ glyco- Edited by Sergio Papa, Gianfranco Gilardi hydrolases (NADases). Members of these families serve important regulatory functions in various and Wilhelm Just cellular compartments, e.g., by linking the cellular energy state to gene expression in the nucleus, by regulating nitrogen metabolism in mitochondria, and by sensing tissue damage in the extracel- + Keywords: lular compartment. Distinct NAD pools may be crucial for these processes. Here, we review the cur- + NAD+ rent knowledge about the compartmentation and biochemistry of NAD -converting enzymes that + ADP-ribosyltransferase control NAD signalling. Sirtuins Ó 2011 Federation of European Biochemical Societies. -

Metabolic Regulation of Epigenetic Modifications and Cell
cancers Review Metabolic Regulation of Epigenetic Modifications and Cell Differentiation in Cancer Pasquale Saggese 1, Assunta Sellitto 2 , Cesar A. Martinez 1, Giorgio Giurato 2 , Giovanni Nassa 2 , Francesca Rizzo 2 , Roberta Tarallo 2 and Claudio Scafoglio 1,* 1 Division of Pulmonary and Critical Care Medicine, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA 90095, USA; [email protected] (P.S.); [email protected] (C.A.M.) 2 Laboratory of Molecular Medicine and Genomics, Department of Medicine, Surgery and Dentistry ‘Scuola Medica Salernitana’, University of Salerno, 84081 Baronissi (SA), Italy; [email protected] (A.S.); [email protected] (G.G.); [email protected] (G.N.); [email protected] (F.R.); [email protected] (R.T.) * Correspondence: [email protected]; Tel.: +1-310-825-9577 Received: 20 October 2020; Accepted: 11 December 2020; Published: 16 December 2020 Simple Summary: Cancer cells change their metabolism to support a chaotic and uncontrolled growth. In addition to meeting the metabolic needs of the cell, these changes in metabolism also affect the patterns of gene activation, changing the identity of cancer cells. As a consequence, cancer cells become more aggressive and more resistant to treatments. In this article, we present a review of the literature on the interactions between metabolism and cell identity, and we explore the mechanisms by which metabolic changes affect gene regulation. This is important because recent therapies under active investigation target both metabolism and gene regulation. The interactions of these new therapies with existing chemotherapies are not known and need to be investigated. -
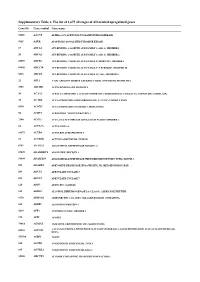
Supplementary Table 1. the List of 1,675 All Stages of AD-Related Upregulated Genes
Supplementary Table 1. The list of 1,675 all stages of AD-related upregulated genes Gene ID Gene symbol Gene name 51146 A4GNT ALPHA-1,4-N-ACETYLGLUCOSAMINYLTRANSFERASE 9625 AATK APOPTOSIS-ASSOCIATED TYROSINE KINASE 19 ABCA1 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 1 20 ABCA2 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 2 10058 ABCB6 ATP-BINDING CASSETTE, SUB-FAMILY B (MDR/TAP), MEMBER 6 89845 ABCC10 ATP-BINDING CASSETTE, SUB-FAMILY C (CFTR/MRP), MEMBER 10 5826 ABCD4 ATP-BINDING CASSETTE, SUB-FAMILY D (ALD), MEMBER 4 25 ABL1 V-ABL ABELSON MURINE LEUKEMIA VIRAL ONCOGENE HOMOLOG 1 3983 ABLIM1 ACTIN BINDING LIM PROTEIN 1 30 ACAA1 ACETYL-COENZYME A ACYLTRANSFERASE 1 (PEROXISOMAL 3-OXOACYL-COENZYME A THIOLASE) 35 ACADS ACYL-COENZYME A DEHYDROGENASE, C-2 TO C-3 SHORT CHAIN 8310 ACOX3 ACYL-COENZYME A OXIDASE 3, PRISTANOYL 56 ACRV1 ACROSOMAL VESICLE PROTEIN 1 2180 ACSL1 ACYL-COA SYNTHETASE LONG-CHAIN FAMILY MEMBER 1 86 ACTL6A ACTIN-LIKE 6A 93973 ACTR8 ACTIN-RELATED PROTEIN 8 91 ACVR1B ACTIVIN A RECEPTOR, TYPE IB 8747 ADAM21 ADAM METALLOPEPTIDASE DOMAIN 21 27299 ADAMDEC1 ADAM-LIKE, DECYSIN 1 56999 ADAMTS9 ADAM METALLOPEPTIDASE WITH THROMBOSPONDIN TYPE 1 MOTIF, 9 105 ADARB2 ADENOSINE DEAMINASE, RNA-SPECIFIC, B2 (RED2 HOMOLOG RAT) 109 ADCY3 ADENYLATE CYCLASE 3 113 ADCY7 ADENYLATE CYCLASE 7 120 ADD3 ADDUCIN 3 (GAMMA) 125 ADH1C ALCOHOL DEHYDROGENASE 1A (CLASS I), ALPHA POLYPEPTIDE 9370 ADIPOQ ADIPONECTIN, C1Q AND COLLAGEN DOMAIN CONTAINING 165 AEBP1 AE BINDING PROTEIN 1 4299 AFF1 AF4/FMR2 FAMILY, MEMBER 1 173 AFM AFAMIN 79814 AGMAT AGMATINE -

Global Profiling of Metabolic Adaptation to Hypoxic Stress in Human Glioblastoma Cells
Global profiling of metabolic adaptation to hypoxic stress in human glioblastoma cells. Kucharzewska, Paulina; Christianson, Helena; Belting, Mattias Published in: PLoS ONE DOI: 10.1371/journal.pone.0116740 2015 Link to publication Citation for published version (APA): Kucharzewska, P., Christianson, H., & Belting, M. (2015). Global profiling of metabolic adaptation to hypoxic stress in human glioblastoma cells. PLoS ONE, 10(1), [e0116740]. https://doi.org/10.1371/journal.pone.0116740 Total number of authors: 3 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 07. Oct. 2021 RESEARCH ARTICLE Global Profiling of Metabolic Adaptation to Hypoxic Stress in Human Glioblastoma Cells Paulina Kucharzewska1, Helena C. -

Epigenetic Regulation in B-Cell Maturation and Its Dysregulation in Autoimmunity
OPEN Cellular and Molecular Immunology (2018) 15, 676–684 www.nature.com/cmi REVIEW Epigenetic regulation in B-cell maturation and its dysregulation in autoimmunity Haijing Wu1, Yaxiong Deng1, Yu Feng1, Di Long1, Kongyang Ma2, Xiaohui Wang2, Ming Zhao1, Liwei Lu2 and Qianjin Lu1 B cells have a critical role in the initiation and acceleration of autoimmune diseases, especially those mediated by autoantibodies. In the peripheral lymphoid system, mature B cells are activated by self or/and foreign antigens and signals from helper T cells for differentiating into either memory B cells or antibody-producing plasma cells. Accumulating evidence has shown that epigenetic regulations modulate somatic hypermutation and class switch DNA recombination during B-cell activation and differentiation. Any abnormalities in these complex regulatory processes may contribute to aberrant antibody production, resulting in autoimmune pathogenesis such as systemic lupus erythematosus. Newly generated knowledge from advanced modern technologies such as next-generation sequencing, single-cell sequencing and DNA methylation sequencing has enabled us to better understand B-cell biology and its role in autoimmune development. Thus this review aims to summarize current research progress in epigenetic modifications contributing to B-cell activation and differentiation, especially under autoimmune conditions such as lupus, rheumatoid arthritis and type 1 diabetes. Cellular and Molecular Immunology advance online publication, 29 January 2018; doi:10.1038/cmi.2017.133 Keywords: -

Enzymes Important in the KREBS-HENSELEIT UREA SYNTHESIZING CYCLE* ARGINASE ORNITHINE CARBAMOYLTRANSFERASE (E.C. 3.5,3,1) from Beef Liver (E.C
enzymes important in the KREBS-HENSELEIT UREA SYNTHESIZING CYCLE* ARGINASE ORNITHINE CARBAMOYLTRANSFERASE (E.C. 3.5,3,1) from Beef Liver (E.C. 2.1.3.3) from Streptococcus faecalis (L-Arginine Ureohydrolase; (Ornithine Transcarbamylase; Carbamoylphosphate: L-Arginine amidinohydrolase) L-Ornithine Carbamoyltransferase) Lyophilized powder Lyophilized with Trizma ® buffer. Catalyzes the following reaction: SuitableforthedeterminationofCarbamyl Phosphate L-Arginine + H~O-*L-Ornithine + Urea and Ornithine, One unit will convert approximately one micromole One unit will form one micromole of Citrulline from of L(+) Arginine to L-Ornithine and Urea per minute Ornithine and Carbamyl Phosphate I~er minute at at pH 9.5 at 37°C. pH 8.5 at 37°C. Order Sigma Product No. A-2137 Order Sigma Product No. O-2501 Approx. 60 units per mg. Protein (Biuret) Approx. 600 units per mg. Protein (Lowry) 2500 units $ 4.85 12,500 units $20.00 30 units $5.00 300 units $40.00 6250 units 11.00 25,000 units 30.00 *Ref.: Krebs, H. A. and Henseleit, K., Z. Physiol. Chem., 210:33 ().932). Other enzymes related to AMMONIA NITROGEN METABOLISM: L-Asparaginase Glutamic Oxalacetic Transaminase Glutaminase L-Aspartase Glutamic-Pyruvic Transaminase L-Histidase Creatininase Gabase Adenosine Deaminase L-Glutamic Dehydrogenase Useful for assay of'r-Aminobutyric Acid. 5'-AMP Deaminase See the Sigma Catalog for further details. ~//~"~ CARBOXYPEPTIDASE - B (E. C. No. 3.4.2.2.) catalyzes hydrolysis of the basic amino acids Arginine and Lysine from the carboxyl terminal position in polypeptides. One of the major pancreatic proteases, Carboxypeptidase-B also functions in the further degradation of products of tryptic digestion. -

Structure and Function of Metallohydrolases in the Arginase- Deacetylase Family
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2016 Structure and Function of Metallohydrolases in the Arginase- Deacetylase Family Yang Hai University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Biochemistry Commons Recommended Citation Hai, Yang, "Structure and Function of Metallohydrolases in the Arginase-Deacetylase Family" (2016). Publicly Accessible Penn Dissertations. 1753. https://repository.upenn.edu/edissertations/1753 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/1753 For more information, please contact [email protected]. Structure and Function of Metallohydrolases in the Arginase-Deacetylase Family Abstract Arginases and deacetylases are metallohydrolases that catalyze two distinct chemical transformations. The arginases catalyze the hydrolysis of the guanidinium group of arginine by using a hydroxide ion 2+ 2+ bridging the binuclear manganese cluster (Mn A-Mn B) for nucleophilic attack. The deacetylases catalyze the hydrolysis of amide bonds by using a mononuclear Zn2+-ion activated water molecule as the nucleophile. Despite the diverse functions, metallohydrolases of the arginase-deacetylase superfamily 2+ share the same characteristic α/β hydrolase core fold and a conserved metal binding site (the Mn B site in arginase corresponds to the catalytic Zn2+ site in deacetylase) which is essential for catalysis in both enzymes. We report crystal structure of formiminoglutamase from the parasitic protozoan Trypanosoma cruzi and confirm that formiminoglutamase is a Mn2+-requiring hydrolase that belongs to the arginase- deacetylase superfamily. We also report the crystal structure of an arginase-like protein from Trypanosoma brucei (TbARG) with unknown function. Although its biological role remains enigmatic, the 2+ evolutionarily more conserved Mn B site can be readily restored in TbARG through side-directed mutagenesis. -

Activation of the AMPK/Sirt1 Pathway by a Leucine
METABOLISM CLINICAL AND EXPERIMENTAL 65 (2016) 1679– 1691 Available online at www.sciencedirect.com Metabolism www.metabolismjournal.com Activation of the AMPK/Sirt1 pathway by a leucine–metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans Jheelam Banerjee⁎, Antje Bruckbauer, Michael B. Zemel NuSirt BioPharma Inc., 11020 Solway School Road, Knoxville, TN 37931, USA ARTICLE INFO ABSTRACT Article history: Background. We have previously shown leucine (Leu) to activate Sirt1 by lowering its KM for Received 28 January 2016 NAD+, thereby amplifying the effects of other sirtuin activators and improving insulin Accepted 29 June 2016 sensitivity. Metformin (Met) converges on this pathway both indirectly (via AMPK) and by direct activation of Sirt1, and we recently found Leu to synergize with Met to improve insulin Keywords: sensitivity and glycemic control while achieving ~80% dose-reduction in diet-induced obese AMPK mice. Accordingly, we sought here to define the mechanism of this interaction. Sirt1 Methods. Muscle cells C2C12 and liver cells HepG2 were used to test the effect of Met–Leu on Insulin sensitivity Sirt1 activation. Caenorhabditis elegans was used for glucose utilization and life span studies. Leucine Results. Leu (0.5 mmol/L) + Met (50–100 μmol/L) synergistically activated Sirt1 (p < 0.001) at + Metformin low (≤100 μmol/L) NAD levels while Met exerted no independent effect. This was associated with an increase in AMPK and ACC, phosphorylation, and increased fatty acid oxidation, which was prevented by AMPK or Sirt inhibition or silencing. Met–Leu also increased P-IRS1/IRS1 and P-AKT/AKT and in insulin-independent glucose disposal in myotubes (~50%, p < 0.002) evident within 30 min as well as a 60% reduction in insulin EC50.