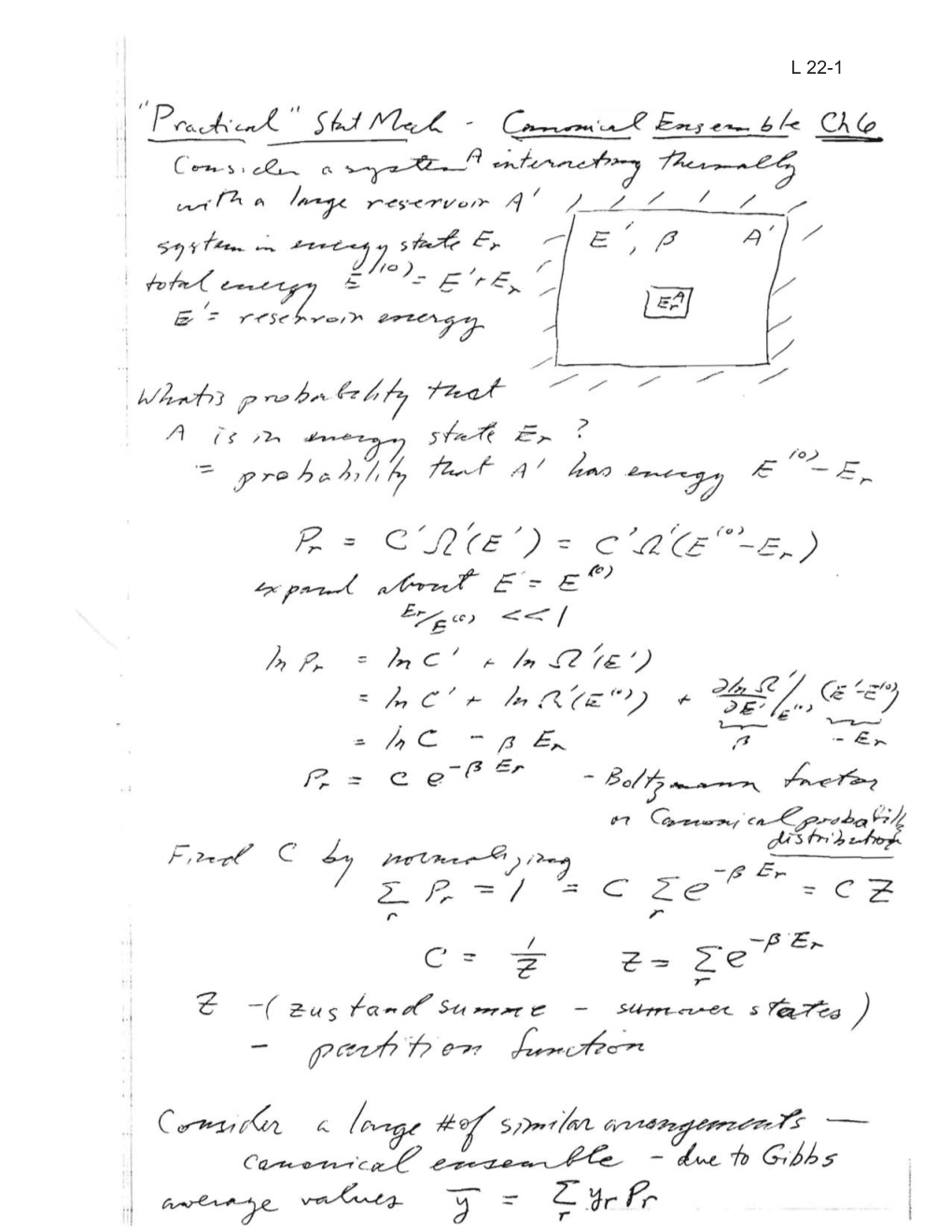
Ph4311 Lecture22.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.On the Sackur-Tetrode Equation in an Expanding Universe
Revista Mexicana de Física ISSN: 0035-001X [email protected] Sociedad Mexicana de Física A.C. México Pereira, S.H. On the Sackur-Tetrode equation in an expanding universe Revista Mexicana de Física, vol. 57, núm. 1, junio, 2011, pp. 11-15 Sociedad Mexicana de Física A.C. Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=57024209002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative ENSENANZA˜ REVISTA MEXICANA DE FISICA´ E 57 (1) 11–15 JUNIO 2011 On the Sackur-Tetrode equation in an expanding universe S.H. Pereira Universidade Federal de Itajuba,´ Campus Itabira, Rua Sao˜ Paulo, 377 – 35900-373, Itabira, MG, Brazil, e-mail: [email protected] Recibido el 5 de abril de 2010; aceptado el 2 de febrero de 2011 In this work we investigate the thermodynamic properties satisfied by an expanding universe filled with a monoatomic ideal gas. We show that the equations for the energy density, entropy density and chemical potential remain the same of an ideal gas confined to a constant volume V . In particular the Sackur-Tetrode equation for the entropy of the ideal gas is also valid in the case of an expanding universe, provided that the constant value that represents the current entropy of the universe is appropriately chosen. Keywords: Expanding universe; ideal gas; Sackur-Tetrode equation. En el presente trabajo investigamos las propriedades termodinamicas´ que son satisfechas por un universo en expansion,´ el cual es lleno por un gas ideal monoatomico.´ Se prueba que las ecuaciones para la densidad de la energ´ıa, la densidad de la entrop´ıa y el potencial qu´ımico son las mismas que las de un gas ideal, el cual se encuentra confinado en un volumen V. -

August/September 2009
August-September 2009 Volume 18, No. 8 TM www.aps.org/publications/apsnews PhysicsQuest APS NEWS Goes to Kenya A PublicAtion of the AmericAn PhysicAl society • www.aps.org/PublicAtions/apsnews See page 5 APS Awards Three Hildred Blewett Scholarships Presidents Two This year APS has announced electrical properties. three women as recipients of the “It was amazing how many ses- M. Hildred Blewett scholarship. sions there were on graphene at the Chosen by the Committee on March Meeting,” Guikema said. the Status of Women in Physics, She plans to use funds from the three are Janice Guikema at the Blewett Scholarship to further Johns Hopkins University, Marija research the feasibility of using Nikolic-Jaric at the University of graphene as a sensitive magnetic Manitoba, and Klejda Bega at Co- detector. She said that graphene has lumbia University. a lot of potential for use as a Hall Each year the committee se- effect detector to detect nanoscale lects women who are returning to particles and map out magnetic their research careers that had been Janice guikema structures. Currently she is continu- interrupted for family or other rea- ing to look for ways to make the sons. The scholarship is a one-year as a second-time recipient of the material as sensitive as possible. grant of up to $45,000 that can be Blewett Scholarship. She currently In addition she will use scanning used towards a wide range of ne- has a part-time research position at probe microscopy to further ex- cessities, including equipment pro- Johns Hopkins University, where plore the nature of graphene. -

MOTION MOUNTAIN the Adventure of Physics – Vol.Iv Quantum Theory: the Smallest Change
Christoph Schiller MOTION MOUNTAIN the adventure of physics – vol.iv quantum theory: the smallest change www.motionmountain.net Christoph Schiller Motion Mountain The Adventure of Physics Volume IV Quantum Theory: The Smallest Change Edition 24.1, available as free pdf at www.motionmountain.net Editio vicesima tertia. Proprietas scriptoris © Christophori Schiller secundo anno Olympiadis vicesimae nonae. Omnia proprietatis iura reservantur et vindicantur. Imitatio prohibita sine auctoris permissione. Non licet pecuniam expetere pro aliquo, quod partem horum verborum continet; liber pro omnibus semper gratuitus erat et manet. Twenty-third edition, ISBN 978-300-021946-7. Copyright © 2009 by Christoph Schiller, the second year of the 29th Olympiad. This pdf file is licensed under the Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 Germany Licence,whosefulltextcanbefoundonthewebsite creativecommons.org/licenses/by-nc-nd/3.0/de, with the additional restriction that reproduction, distribution and use, in whole or in part, in any product or service, be it commercial or not, is not allowed without the written consent of the copyright owner. The pdf file was and remains free for everybody to read, store and print for personal use, and to distribute electronically, but only in unmodified form and at no charge. To Britta, Esther and Justus Aaron τῷ ἐμοὶ δαὶμονι Die Menschen stärken, die Sachen klären. PREFACE Primum movere, deinde docere.* Antiquity “ ” Motion Mountain – The Adventure of Physics pdf file available free of charg This book is written for anybody who is curious about nature and motion. Have you ever asked: Why do people, animals, things, images and space move? The answer leads to many adventures; this volume presents those due the discovery that there is a smallest change in nature. -

Planck's Quantum Theory of Ideal
Pursuing an Idea: Planck’s Quantum Theory of Ideal Gas ∗ Massimiliano Badino† 1 Prelude: One Hundred Years of ‘Quantitude’ Many years later, as he faced the firing squad, Erwin Planck was to remember that distant afternoon when his father took him to discover the quantum. It was indeed in a day at the beginning of the last century, during a walk in the Grunewald Forest, that Max Planck in- formed his youngest son that his recent discovery was the greatest since the time of Newton. After that day, physics was never the same again. From the mysterious realm of the interac- tion between matter and radiation, the quantum spread out both in the theory of radiation and in the theory of matter and eventually changed our picture of the physical world. Planck played a foremost role in this revolution till the mid-1920 and especially his work on the ap- plication of quantum hypothesis to the ideal gas was instrumental in setting the stage for quantum mechanics. In this paper I will give a cursory outline of this work and, centering on the argumen- tative structure, I will argue that Planck’s theory of gas was framed according to a theoretical strategy he had already adopted in his previous work on radiation theory. The thrust of this strategy consists in focussing on the general features of the system and leaving aside spe- cific assumptions on the micro-processes. Throughout the years Planck reconfigured and reorganized his arguments and the great flexibility of his theoretical strategy allowed him to maintain a consistent outlook on the problem. -

Otto Stern Annalen 22.9.11
September 22, 2011 Otto Stern (1888-1969): The founding father of experimental atomic physics J. Peter Toennies,1 Horst Schmidt-Böcking,2 Bretislav Friedrich,3 Julian C.A. Lower2 1Max-Planck-Institut für Dynamik und Selbstorganisation Bunsenstrasse 10, 37073 Göttingen 2Institut für Kernphysik, Goethe Universität Frankfurt Max-von-Laue-Strasse 1, 60438 Frankfurt 3Fritz-Haber-Institut der Max-Planck-Gesellschaft Faradayweg 4-6, 14195 Berlin Keywords History of Science, Atomic Physics, Quantum Physics, Stern- Gerlach experiment, molecular beams, space quantization, magnetic dipole moments of nucleons, diffraction of matter waves, Nobel Prizes, University of Zurich, University of Frankfurt, University of Rostock, University of Hamburg, Carnegie Institute. We review the work and life of Otto Stern who developed the molecular beam technique and with its aid laid the foundations of experimental atomic physics. Among the key results of his research are: the experimental determination of the Maxwell-Boltzmann distribution of molecular velocities (1920), experimental demonstration of space quantization of angular momentum (1922), diffraction of matter waves comprised of atoms and molecules by crystals (1931) and the determination of the magnetic dipole moments of the proton and deuteron (1933). 1 Introduction Short lists of the pioneers of quantum mechanics featured in textbooks and historical accounts alike typically include the names of Max Planck, Albert Einstein, Arnold Sommerfeld, Niels Bohr, Werner Heisenberg, Erwin Schrödinger, Paul Dirac, Max Born, and Wolfgang Pauli on the theory side, and of Konrad Röntgen, Ernest Rutherford, Max von Laue, Arthur Compton, and James Franck on the experimental side. However, the records in the Archive of the Nobel Foundation as well as scientific correspondence, oral-history accounts and scientometric evidence suggest that at least one more name should be added to the list: that of the “experimenting theorist” Otto Stern. -

TERMODYNAMIK En Kort Historik Christoffer Norberg
ISRN LUTMDN/TMHP-08/3032-SE ISSN 0282-1990 Institutionen f¨or Energivetenskaper TERMODYNAMIK en kort historik Christoffer Norberg Joules skovelanordning fr˚an 1845/7 f¨or att best¨amma den mekaniska v¨armeekvivalenten. Phil. Trans. Roy. Soc. 140 (1850). januari 2008 F¨orord Denna skrift g¨or inga anspr˚ak p˚aatt vara komplett eller utt¨ommande. D¨aremot har jag i m¨ojligaste m˚an f¨ors¨okt vara korrekt n¨ar det g¨aller ˚artal, biografiska data och prioritet av originalarbeten. F¨or en mer utt¨ommande beskrivning (fram till 1800-talets slut) re- kommenderas From Watt to Clausius av Donald Cardwell ([5]). Kommentarer och f¨orslag till korrigeringar emottages tacksamt. Portr¨att ¨ar huvudsakligen h¨amtade fr˚an Internet samt [2, 6, 25, 28, 20, 27], biografiska data v¨asentligen ur [1, 28, 7, 12, 21, 26, 30] och originalreferenser mestadels ur bibliotekss¨okningar, tillg¨angliga tidskrifter inom LU-n¨atet samt [23, 30]. 8 januari 20081 Christoffer Norberg Tel. 046-2228606 Christoff[email protected] Levnads˚ar f¨or 35 pionj¨arer inom termodynamikens historiska utveckling. Tjocka linjer motsvarar ˚aldern 20–65 ˚ar. “But although, as a matter of history, statistical mechanics owes its origin to investigations in thermodynamics, it seems eminently worthy of an independent development, both on account of the elegance and simplicity of its principles, and because it yields new results and places old truths in a new light in departments quite outside of thermodynamics.” Willard Gibbs 1Sedan tryckningen fr˚an januari 2008 har det gjorts ett par uppdateringar av biografiska data, liksom sm¨arre justeringar och till¨agg i texten samt i den bibliografiska delen; 15 december 2013. -

On the 100Th Anniversary of the Sackur–Tetrode Equation
On the 100th Anniversary of the Sackur–Tetrode Equation Walter Grimus (University of Vienna) Seminar Particle Physics March 8, 2012, University of Vienna Walter Grimus (University of Vienna) On the 100th Anniversary of the Sackur–Tetrode Equation Sackur–Tetrode equation Entropy of a monoatomic ideal gas: 3 E V S(E, V , N)= kN ln + ln + s 2 N N 0 1912: Otto Sackur and Hugo Tetrode independently determined 3 4πm 5 s = ln + 0 2 3h2 2 Sackur–Tetrode equation = absolute entropy of a monoatomic ideal gas Walter Grimus (University of Vienna) On the 100th Anniversary of the Sackur–Tetrode Equation Contents 1 Motivation 2 Tetrode’s derivation 3 Sackur’s derivation 4 Test of the Sackur–Tetrode equation 5 Concluding remarks Walter Grimus (University of Vienna) On the 100th Anniversary of the Sackur–Tetrode Equation Motivation Absolute entropy: Boltzmann (1875), Planck (1900): S = k ln W + const. Argument: Nernst’s heat theorem (1906) (third law of thermodynamics) S should be calculable without any additive constant ⇒ Massive particles: phase space volume of “elementary cells” unknown Sackur (1911): Entropy of a monoatomic ideal gas as a function of the volume of elementary cell Walter Grimus (University of Vienna) On the 100th Anniversary of the Sackur–Tetrode Equation Tetrode’s derivation Ansatz: n degrees of freedom ⇒ n Elementary domains of volume σ = δq δp δqn δpn = (zh) 1 1 · · · N identical particles ⇒ W ′ S = k ln N! W ′ = number of configurations in phase space Total admissible volume in phase space space given E, V , N: 3 3 3 3 (E, V , N)= -

On the 100Th Anniversary of the Sackur-Tetrode Equation
UWThPh-2011-34 On the 100th anniversary of the Sackur–Tetrode equation W. Grimus∗ University of Vienna, Faculty of Physics Boltzmanngasse 5, A–1090 Vienna, Austria 23 January 2013 Abstract In 1912, Otto Sackur and Hugo Tetrode independently put forward an equation for the absolute entropy of a monoatomic ideal gas and published it in “Annalen der Physik.” The grand achievement in the derivation of this equation was the discretization of phase space for massive particles, expressed as δqδp = h, where q and p are conjugate variables and h is Planck’s constant. Due to the dependence of the absolute entropy on Planck’s constant, Sackur and Tetrode were able to devise a test of their equation by applying it to the monoatomic vapor of mercury; from the satisfactory numerical comparison of h obtained from thermodynamic data on mercury with Planck’s value from black-body radiation, they inferred the correctness of their equation. In this review we highlight this almost forgotten episode of physics, discuss the arguments leading to the derivation of the Sackur–Tetrode equation and outline the method how this equation was tested with thermodynamic data. arXiv:1112.3748v2 [physics.hist-ph] 23 Jan 2013 PACS: 05.70.-a, 51.30.+i ∗E-mail: [email protected] 1 1 Introduction The formula for the absolute entropy of a monoatomic ideal gas is named after Otto Sackur and Hugo Tetrode who independently derived it in 1912 [1, 2, 3]. In classical thermodynamics the entropy of a monoatomic ideal gas is 3 E V S(E,V,N)= kN ln + ln + s0 , (1) 2 N N where E, V and N are the kinetic energy, the volume and the number of atoms, respec- tively. -

One Hundred Years of Chemical Warfare: Research
Bretislav Friedrich · Dieter Hoffmann Jürgen Renn · Florian Schmaltz · Martin Wolf Editors One Hundred Years of Chemical Warfare: Research, Deployment, Consequences One Hundred Years of Chemical Warfare: Research, Deployment, Consequences Bretislav Friedrich • Dieter Hoffmann Jürgen Renn • Florian Schmaltz Martin Wolf Editors One Hundred Years of Chemical Warfare: Research, Deployment, Consequences Editors Bretislav Friedrich Florian Schmaltz Fritz Haber Institute of the Max Planck Max Planck Institute for the History of Society Science Berlin Berlin Germany Germany Dieter Hoffmann Martin Wolf Max Planck Institute for the History of Fritz Haber Institute of the Max Planck Science Society Berlin Berlin Germany Germany Jürgen Renn Max Planck Institute for the History of Science Berlin Germany ISBN 978-3-319-51663-9 ISBN 978-3-319-51664-6 (eBook) DOI 10.1007/978-3-319-51664-6 Library of Congress Control Number: 2017941064 © The Editor(s) (if applicable) and The Author(s) 2017. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution-NonCommercial 2.5 International License (http://creativecommons.org/licenses/by-nc/2.5/), which permits any noncom- mercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. -

A Equação De Sackur Tetrode Ou Como a Entropia Se Encontrou Com
A equação de Sackur-Tetrode ou como a entropia se encontrou com a mecânica quântica 3=2 5=2 2 3=2 5=2 S = Nk ln M T =P + ln 2=NAvh k + 5=2 n h i o No início do século XX, cientistas ilustres lutavam para entender mais profundamente o conceito de entropia. Esse era o conceito central da toda poderosa “segunda lei da termodinâmica”, que já tinha sido utilizada para estabelecer uma escala absoluta de temperaturas, mas que ainda demandava boa dose de esclarecimento. Uma questão inquietante permanecia sem resposta. Será que o valor ab- soluto da entropia poderia ser determinado? Ou será que a entropia somente poderia ser determinada a menos de uma constante aditiva? Os cientistas daquela época passaram a prestar maior atenção às ideias de Ludwig Boltzmann. O extenso trabalho de Boltzmann nesse problema pode ser resumido pela inscrição no seu túmulo, no cemitério central de Viena, S = k ln W: Essa equação expressa a entropia S em termos do logaritmo do número W de estados disponíveis para o movimento, multiplicado pelo constante k, batizada mais tarde em homenagem a Boltzmann. No entanto, de acordo com a física clássica, não há limite para a proximidade dos estados mecânicos no espaço de fase. Portanto, não há limite para o número de estados, e nem seria possível enumerá-los. Como é então que seria possível obter um valor de W bem de…nido? Como é que obteríamos a constante aditiva da entropia? A resposta a essas questões foi dada em dois artigos separados, que apare- ceram nos Annalen der Physik, principal revista alemã de física. -

The Gibbs Paradox: Early History and Solutions
entropy Article The Gibbs Paradox: Early History and Solutions Olivier Darrigol UMR SPHere, CNRS, Université Denis Diderot, 75013 Paris, France; [email protected] Received: 9 April 2018; Accepted: 28 May 2018; Published: 6 June 2018 Abstract: This article is a detailed history of the Gibbs paradox, with philosophical morals. It purports to explain the origins of the paradox, to describe and criticize solutions of the paradox from the early times to the present, to use the history of statistical mechanics as a reservoir of ideas for clarifying foundations and removing prejudices, and to relate the paradox to broad misunderstandings of the nature of physical theory. Keywords: Gibbs paradox; mixing; entropy; irreversibility; thermochemistry 1. Introduction The history of thermodynamics has three famous “paradoxes”: Josiah Willard Gibbs’s mixing paradox of 1876, Josef Loschmidt reversibility paradox of the same year, and Ernst Zermelo’s recurrence paradox of 1896. The second and third revealed contradictions between the law of entropy increase and the properties of the underlying molecular dynamics. They prompted Ludwig Boltzmann to deepen the statistical understanding of thermodynamic irreversibility. The Gibbs paradox—first called a paradox by Pierre Duhem in 1892—denounced a violation of the continuity principle: the mixing entropy of two gases (to be defined in a moment) has the same finite value no matter how small the difference between the two gases, even though common sense requires the mixing entropy to vanish for identical gases (you do not really mix two identical substances). Although this paradox originally belonged to purely macroscopic thermodynamics, Gibbs perceived kinetic-molecular implications and James Clerk Maxwell promptly followed him in this direction. -

James, Steinhauser, Hoffmann, Friedrich One Hundred Years at The
James, Steinhauser, Hoffmann, Friedrich One Hundred Years at the Intersection of Chemistry and Physics Published under the auspices of the Board of Directors of the Fritz Haber Institute of the Max Planck Society: Hans-Joachim Freund Gerard Meijer Matthias Scheffler Robert Schlögl Martin Wolf Jeremiah James · Thomas Steinhauser · Dieter Hoffmann · Bretislav Friedrich One Hundred Years at the Intersection of Chemistry and Physics The Fritz Haber Institute of the Max Planck Society 1911–2011 De Gruyter An electronic version of this book is freely available, thanks to the support of libra- ries working with Knowledge Unlatched. KU is a collaborative initiative designed to make high quality books Open Access. More information about the initiative can be found at www.knowledgeunlatched.org Aut ho rs: Dr. Jeremiah James Prof. Dr. Dieter Hoffmann Fritz Haber Institute of the Max Planck Institute for the Max Planck Society History of Science Faradayweg 4–6 Boltzmannstr. 22 14195 Berlin 14195 Berlin [email protected] [email protected] Dr. Thomas Steinhauser Prof. Dr. Bretislav Friedrich Fritz Haber Institute of the Fritz Haber Institute of the Max Planck Society Max Planck Society Faradayweg 4–6 Faradayweg 4–6 14195 Berlin 14195 Berlin [email protected] [email protected] Cover images: Front cover: Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry, 1913. From left to right, “factory” building, main building, director’s villa, known today as Haber Villa. Back cover: Campus of the Fritz Haber Institute of the Max Planck Society, 2011. The Institute’s his- toric buildings, contiguous with the “Röntgenbau” on their right, house the Departments of Physical Chemistry and Molecular Physics.