Chemistry (CHEM) 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -

Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A
Article pubs.acs.org/joc Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A. Glover* and Adam A. Rosser Department of Chemistry, School of Science and Technology, University of New England, Armidale, NSW 2351, Australia *S Supporting Information ABSTRACT: Two independent computational methods have been used for determination of amide resonance stabilization and amidicities relative to N,N-dimethylacetamide for a wide range of acyclic and cyclic amides. The first method utilizes carbonyl substitution nitrogen atom replacement (COSNAR). The second, new approach involves determination of the difference in amide resonance between N,N-dimethylacetamide and the target amide using an isodesmic trans-amidation process and is calibrated relative to 1-aza-2-adamantanone with zero amidicity and N,N-dimethylace- tamide with 100% amidicity. Results indicate excellent coherence between the methods, which must be regarded as more reliable than a recently reported approach to amidicities based upon enthalpies of hydrogenation. Data for acyclic planar and twisted amides are predictable on the basis of the degrees of pyramidalization at nitrogen and twisting about the C−N bonds. Monocyclic lactams are predicted to have amidicities at least as high as N,N-dimethylacetamide, and the β-lactam system is planar with greater amide resonance than that of N,N- dimethylacetamide. Bicyclic penam/em and cepham/em scaffolds lose some amidicity in line with the degree of strain-induced pyramidalization at the bridgehead nitrogen and twist about the amide bond, but the most puckered penem system still retains substantial amidicity equivalent to 73% that of N,N-dimethylacetamide. ■ INTRODUCTION completed by a third resonance form, III, with no C−N pi Amide linkages are ubiquitous in proteins, peptides, and natural character and on account of positive polarity at carbon and the or synthetic molecules and in most of these they possess, electronegativity of nitrogen, must be destabilizing. -

Faculty of Science Internal Bylaw of Graduate Studies "Program Curricula and Course Contents" 2016
. Faculty of Science Internal Bylaw of Graduate Studies "Program Curricula and Course Contents" 2016 Table of Contents Page 1-Mathematics Department Mathematics Programs 1 Diplomas Professional Diploma in Applied Statistics 2 Professional Diploma in Bioinformatics 3 M.Sc. Degree M.Sc. Degree in Pure Mathematics 4 M.Sc. Degree in Applied Mathematics 5 M.Sc. Degree in Mathematical Statistics 6 M.Sc. Degree in Computer Science 7 M.Sc. Degree in Scientific Computing 8 Ph.D. Degree Ph. D. Degree in Pure Mathematics 9 Ph. D Degree in Applied Mathematics 10 Ph. D. Degree in Mathematical Statistics 11 Ph. D Degree in Computer Science 12 Ph. D Degree in Scientific Computing 13 2- Physics Department Physics Programs 14 Diplomas Diploma in Medical Physics 15 M.Sc. Degree M.Sc. Degree in Solid State Physics 16 M.Sc. Degree in Nanomaterials 17 M.Sc. Degree in Nuclear Physics 18 M.Sc. Degree in Radiation Physics 19 M.Sc. Degree in Plasma Physics 20 M.Sc. Degree in Laser Physics 21 M.Sc. Degree in Theoretical Physics 22 M.Sc. Degree in Medical Physics 23 Ph.D. Degree Ph.D. Degree in Solid State Physics 24 Ph.D. Degree in Nanomaterials 25 Ph.D. Degree in Nuclear Physics 26 Ph.D. Degree in Radiation Physics 27 Ph.D. Degree in Plasma Physics 28 Ph.D. Degree in Laser Physics 29 Ph.D. Degree in Theoretical Physics 30 3- Chemistry Department Chemistry Programs 31 Diplomas Professional Diploma in Biochemistry 32 Professional Diploma in Quality Control 33 Professional Diploma in Applied Forensic Chemistry 34 Professional Diploma in Applied Organic Chemistry 35 Environmental Analytical Chemistry Diploma 36 M.Sc. -

The Birth of Modern Chemistry
The Birth of Modern Chemistry 3rd semester project, Fall 2008 NIB Group nr.5, House 13.2 Thomas Allan Rayner & Aiga Mackevica Supervisor: Torben Brauner Abstract Alchemy was a science practiced for more than two millennia up till the end of 18th century when it was replaced by modern chemistry, which is practiced up till this very day. The purpose of this report is to look into this shift and investigate whether this shift can be classified as a paradigm shift according to the famous philosopher Thomas Kuhn, who came up with a theory on the structure of scientific revolutions. In order to come to draw any kind of conclusions, the report summarizes and defines the criteria for what constitutes a paradigm, crisis and paradigm shift, which are all important in order to investigate the manner in which a paradigm shift occurs. The criteria are applied to the historical development of modern chemistry and alchemy as well as the transition between the two sciences. As a result, alchemy and modern chemistry satisfy the requirements and can be viewed as two different paradigms in a Kuhnian sense. However, it is debatable whether the shift from alchemy to modern chemistry can be called a paradigm shift. 2 Acknowledgments We would like to thank Torben Brauner for his guidance throughout the project period as our supervisor. We would also like to thank our opposing group – Paula Melo Paulon Hansen, Stine Hesselholt Sloth and their supervisor Ole Andersen for their advice and critique for our project. 3 Table of Contents 1. Introduction ................................................................................................................................ 5 2. -

General Chemistry Course Description
Chemistry 110: General Chemistry Note: this is a representative syllabus for Chemistry 110. It is here for informational purposes. It is not intended to substitute for or replace the syllabus your instructor provides. Course Description: Introduction to the general principles of chemistry for students planning a professional career in chemistry, a related science, the health professions, or engineering. Stoichiometry, atomic structure, chemical bonding and geometry, thermochemistry, gases, types of chemical reactions, statistics. Weekly laboratory exercises emphasize quantitative techniques and complement the lecture material. Weekly discussion sessions focus on homework assignments and lecture material. Prerequisite: Mathematics 055 or equivalent. Previous high school chemistry not required. Taught: Annually, 5 semester credits. Course Components: Lecture, Recitation section, Laboratory Textbook: Steven S. Zumdahl and Susan A. Zumdahl, Chemistry, 5th Ed., Houghton Mifflin Company (New York, 2000). The text is available in the college bookstore. Lab Manual: Chemistry Department Faculty, Chemistry 110 Lab Manual, Fall, 2001 Edition, Kinkos. These may be purchased from Linda Noble, the Chemistry Secretary. Safety Goggles: These must be worn at all times in the laboratory. They can be purchased in the college bookstore. Calculator: Must have logs and exponential capability. Lab Notebook: This is a bound duplicate notebook with numbered pages. It can be purchased in the college bookstore Lecture Schedule 1.1-1.5 introduction, laboratory precision -

Course Syllabus
Course Syllabus Department: Science & Technology Date: January 2015 I. Course Prefix and Number: CHM 205 Course Name: Organic Chemistry I – Lecture Only Credit Hours and Contact Hours: 4 credit hours and 4 (3:0:1) contact hours Catalog Description including pre- and co-requisites: A systematic study of the chemistry of carbon compounds emphasizing reactions, mechanisms, and synthesis with a focus on functional groups, addition reactions to alkenes and alkynes, alcohols and ethers, stereochemistry, nomenclature, acid-base chemistry, reaction kinetics and thermodynamics. Completion of General Chemistry II or equivalent with a grade of C or better is prerequisite. II. Course Outcomes and Objectives Student Learning Outcomes: Upon completion of this course, the student will be able to: Demonstrate an understanding of basic principles of organic chemistry and how they relate to everyday experiences. Demonstrate problem solving and critical thinking skills Apply methods of scientific inquiry. Apply problem solving techniques to real-world problems. Demonstrate an understanding of the chemical environment and the role that organic molecules play in the natural and the synthetic world. Relationship to Academic Programs and Curriculum: This course is required for majors in chemistry, chemical engineering, biology, biotechnology, pharmacology, and other pre-professional programs. College Learning Outcomes Addressed by the Course: writing computer literacy oral communications ethics/values X reading citizenship mathematics global concerns X critical thinking information resources 1 III. Instructional Materials and Methods Types of Course Materials: A standard two-semester 200 level organic textbook and workbook. Methods of Instruction (e.g. Lecture and Seminar …): Three hours of lecture, with a one hour recitation period for individual as well as group learning activities such as case studies and guided learning activities. -

CHEMISTRY MODULE No.31 : Magnetic Properties of Transition
Web links http://en.wikipedia.org/wiki/Magnetism http://wwwchem.uwimona.edu.jm/courses/magnetism.html https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/transition- metals-22/bonding-in-coordination-compounds-crystal-field-theory-160/magnetic-properties- 616-6882/ http://en.wikipedia.org/wiki/Transition_metal http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Crystal_Field_Theory/Crystal_Field_Theo ry/Magnetic_Properties_of_Coordination_Complexes Suggested Readings Miessler, G. L.; Tarr, D. A. (2003). Inorganic Chemistry (3rd ed.). Pearson Prentice Hall. ISBN 0-13-035471-6 Drago, R. S.Physical Methods In Chemistry. W.B. Saunders Company. ISBN 0721631843 (ISBN13: 9780721631844) Huheey, J. E.; Keiter, E.A. ; Keiter, R. L. ; Medhi O. K. Inorganic Chemistry: Principles of Structure and Reactivity.Pearson Education India, 2006 - Chemistry, Inorganic CHEMISTRY PAPER No.: 7; Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) MODULE No.31 : Magnetic properties of transition metal ions Carlin, R. L. Magnetochemistry. SPRINGER VERLAG GMBH. ISBN 10: 3642707351 / ISBN 13: 9783642707353 SELWOOD, P. W. MAGNETOCHEMISIRY. Swinburne Press. ISBN 1443724890. Earnshaw, A. Introduction to Magnetochemistry Academic Press. ISBN 10: 1483255239 / ISBN 13: 9781483255231 Lacheisserie É, D. T. De; Gignoux, D., Schlenker, M. Magnetism Time-Lines Timeline Image Description s 1600 Dr. William Gilbert published the first systematic experiments on magnetism in "De Magnete". Source: http://en.wikipedia.org/wiki/William_Gilbert_(astronomer) CHEMISTRY PAPER No.: 7; Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) MODULE No.31 : Magnetic properties of transition metal ions 1777 Charles-Augustin de Coulomb showed that the magnetic repulsion or attraction between magnetic poles varies inversely with the square of the http://en.wikipedia.org/wiki/Charles-Augustin_de_Coulomb distance r. -
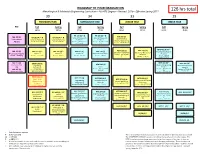
BS Metallurgical Engineering Curriculum Flowchart
ROADMAP TO YOUR GRADUATION Metallurgical & Materials Engineering Curriculum – BS MTE Degree – Revised 2016-- Effective Spring 2017 126 hrs total 30 34 33 29 FRESHMAN YEAR SOPHOMORE YEAR JUNIOR YEAR SENIOR YEAR Pre Fall SprIng Fall SprIng Fall SprIng Fall SprIng 16 hrs 14 hrs 17 hrs 17 hrs 16 hrs 17 hrs 14 hrs 15 hrs PH 105 (4) * N PH 106 (4) * N ECE 320 (3) MA 100 (3) CH 101 (4) * N CH 102 (4) * N General Physics with General Physics with Fundamentals of Intermediate General Chemistry 1 General Chemistry 2 Calculus 1 Calculus 2 Electrical Engineering Pre-reqs. = See catalog Pre-reqs. = CH 101 Pre-reqs. = MA 113 or 115 Pre-reqs. = PH 101 or 105 or Algebra Pre-reqs. = PH 106, MA 238 or 125 or 145 125 MTE 481 (4) W * AEM 250 (3) MTE 455 (4) MA 112 (3) MA 125 (4) * MA 126 (4) * MA 227 (4) MA 238 (3) Analytical Methods for Mechanics of Materials Mechanical Behavior of Precalculus Algebra Calculus 1 Calculus 2 Calculus 3 Differential Equations 1 Materials Pre-reqs. = MA 126, AEM Materials Pre-reqs. = See catalog Pre-reqs. = See catalog Pre-reqs. = MA 125 Pre-reqs. = MA 126 Pre-reqs. = MA 126 Pre-req. = MA 238 201 Pre-reqs. = AEM 250 Co-req. = MTE 441 # MA 113 (3) ENGR 103 (3) MTE 443 (3) MTE 445 (3) # AEM 201 (3) Materials Engineering Precalculus Trig Engineering Materials Engineering Statics Design 1 OR Foundations Design 2 Pre-reqs. = MA 125, PH 105, Pre-reqs. = MTE 362, 373, Pre-reqs. = MATH 125 or Pre-reqs. -

Professor of Chemistry
Bruce C. Gibb FRSC Professor of Chemistry Department of Chemistry Tulane University New Orleans, LA 70118, USA Tel: (504) 862 8136 E-mail: [email protected] Website: http://www.gibbgroup.org Twitter: @brucecgibb Research Interests: Aqueous supramolecular chemistry: understanding how molecules interact in water: from specific ion- pairing and the hydrophobic effect, to protein aggregation pertinent to neurodegenerative disorders. Our research has primarily focused on: 1) novel hosts designed to probe the hydrophobic, Hofmeister, and Reverse Hofmeister effects, and; 2) designing supramolecular capsules as yocto-liter reaction vessels and separators. Current efforts to probe the hydrophobic and Hofmeister effects include studies of the supramolecular properties of proteins. Professional Positions: Visiting Professor, Wuhan University of Science and Technology as a Chair Professor of Chutian Scholars Program (2015-2018) Professor of Chemistry, Tulane University, New Orleans, USA (2012-present). University Research Professor, University of New Orleans, USA (2007-2011). Professor of Chemistry, University of New Orleans, USA (2005-2007). Associate Professor of Chemistry, University of New Orleans, USA (2002-2005). Assistant Professor of Chemistry, University of New Orleans, USA, (1996-2002). Education: Postdoctoral Work Department of Chemistry, New York University. Synthesis of Carbonic Anhydrase (CA) mimics with Advisor: Prof. J. W. Canary, (1994-1996). Department of Chemistry, University of British Columbia, Canada De Novo Protein development. Advisor: Prof. J. C. Sherman (1993-1994). Ph.D. Robert Gordon’s University, Aberdeen, UK. Synthesis and Structural Examination of 3a,5-cyclo-5a- Androstane Steroids. Advisors: Dr. Philip J. Cox and Dr. Steven MacManus (1987-92) . B.Sc. with Honors in Physical Sciences Robert Gordon’s University, Aberdeen, UK. -

Department of Chemistry Faculty of Science
M.Phil./Ph.D. Syllabus DEPARTMENT OF CHEMISTRY FACULTY OF SCIENCE THE UNIVERSITY OF RAJSHAHI Syllabus for The Degree of Master of Philosophy (M.Phil.)/ Doctor of Philosophy (Ph.D.) in Chemistry Session: 2017-2018 Department of Chemistry, University of Rajshahi January, 2018 DEPARTMENT OF CHEMISTRY Syllabus for M. Phil / Ph.D. Courses The following courses each of 100 marks are designed for M..Phil / Ph.D. students as per ordinance passed by the Academic Council held on 17-18/10-98 (decision no. 37 of 196th Academic Council) and approved by the Syndicate at its 351st meeting held on 22/10/98. Course Number Course Title Chem 611 Physical Chemistry - X Chem 612 Physical Chemistry - XI Chem 613 Physical Chemistry - XII Chem 621 Organic Chemistry - VIII Chem 622 Organic Chemistry - IX Chem 623 Industrial Chemistry - II Chem 631 Inorganic Chemistry - IX Chem 632 Inorganic Chemistry - X Chem 633 Analytical Chemistry – II Chem 634 Bioinorganic Chemistry * Marks Distribution: (a) Close Book Examination : 75 (b) Class Assessment : 25 A student must complete two courses out of the above ten courses. The courses will be assigned to a research fellow on the recommendation of supervisor(s) and approved by the departmental Chairman / Academic Committee. 1 Course : Chem 611 Physical Chemistry-X Examination : 4 hours Full marks : 100 1. Physical Properties of Gases: Kinetic molecular theory, equation of states and transport phenomena of gases. 2. Chemical Thermodynamics: Thermochemistry, statistical thermodynamics, non-equilibrium or reversible thermodynamics. 3. Chemical Equilibria, Phase Rule and Distribution law. 4. Electrochemistry:Electrolytic conductance, electrolytic transference and Ionic equilibria. 5. -

Chemistry (CH) 1
Chemistry (CH) 1 Chemistry (CH) CH 101 - INTRO TO CHEMISTRY Semester Hours: 3 Properties of solids, liquids, gases, and solutions, atomic theory and bonding, concentration concepts, and physical and chemical properties of the more common elements and their compounds. No placement examination is required. Prerequisite: MA 110 or prerequisites with concurrency MA 112 or higher and CH 105. CH 101R - RECITATION Semester Hours: 0 CH 105 - INTRO CHEMISTRY LAB Semester Hour: 1 Complements the lecture material for CH 101. Laboratory fundamentals and basic chemical principles. Prerequisite with concurrency: CH 101. CH 121 - GENERAL CHEMISTRY I Semester Hours: 3 For science and engineering majors. Chemical properties of elements, their periodic groups, and their compounds. Reactions and stoichiometry. Nature of the chemical bond, molecular structure, thermochemistry. Properties of gases, liquids, and solids. Prerequisite: CH 101 or placement test. Prerequisites with concurrency: MA 113 or higher, and CH 125. CH 121M - GENERAL CHEMISTRY FOR CHEMISTS Semester Hours: 3 For Chemistry Majors and Minors. Chemical properties of elements and the Periodic Table. Reactions and stoichiometry. Nature of chemical bonds, molecular structure, thermochemistry. Properties of gases, liquids, and solids. Intro to your exciting Department and its programs. Engagement activities. Prerequisites: CH 101 or Placement. Prerequisites with concurrency: MA 113 or higher, CH 125. CH 121R - RECITATION Semester Hours: 0 CH 122 - GENERAL CHEMISTRY ENGINEERS Semester Hours: 3 This course is designed as a one semester presentation of key aspects in general chemistry and is recommended for all engineering majors except chemical engineers. Covers topic on atoms and molecules: reactions and stoichiometry; gases; the periodic table; atomic structure, chemical bonding and molecular structure; materials; energy, entropy, and free energy; kinetics and equilibrium; and electrochemistry.