Introduction to General Chemistry Calculations and Concepts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

500 Natural Sciences and Mathematics
500 500 Natural sciences and mathematics Natural sciences: sciences that deal with matter and energy, or with objects and processes observable in nature Class here interdisciplinary works on natural and applied sciences Class natural history in 508. Class scientific principles of a subject with the subject, plus notation 01 from Table 1, e.g., scientific principles of photography 770.1 For government policy on science, see 338.9; for applied sciences, see 600 See Manual at 231.7 vs. 213, 500, 576.8; also at 338.9 vs. 352.7, 500; also at 500 vs. 001 SUMMARY 500.2–.8 [Physical sciences, space sciences, groups of people] 501–509 Standard subdivisions and natural history 510 Mathematics 520 Astronomy and allied sciences 530 Physics 540 Chemistry and allied sciences 550 Earth sciences 560 Paleontology 570 Biology 580 Plants 590 Animals .2 Physical sciences For astronomy and allied sciences, see 520; for physics, see 530; for chemistry and allied sciences, see 540; for earth sciences, see 550 .5 Space sciences For astronomy, see 520; for earth sciences in other worlds, see 550. For space sciences aspects of a specific subject, see the subject, plus notation 091 from Table 1, e.g., chemical reactions in space 541.390919 See Manual at 520 vs. 500.5, 523.1, 530.1, 919.9 .8 Groups of people Add to base number 500.8 the numbers following —08 in notation 081–089 from Table 1, e.g., women in science 500.82 501 Philosophy and theory Class scientific method as a general research technique in 001.4; class scientific method applied in the natural sciences in 507.2 502 Miscellany 577 502 Dewey Decimal Classification 502 .8 Auxiliary techniques and procedures; apparatus, equipment, materials Including microscopy; microscopes; interdisciplinary works on microscopy Class stereology with compound microscopes, stereology with electron microscopes in 502; class interdisciplinary works on photomicrography in 778.3 For manufacture of microscopes, see 681. -

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -
A New Rule-Based Method of Automatic Phonetic Notation On
A New Rule-based Method of Automatic Phonetic Notation on Polyphones ZHENG Min, CAI Lianhong (Department of Computer Science and Technology of Tsinghua University, Beijing, 100084) Email: [email protected], [email protected] Abstract: In this paper, a new rule-based method of automatic 2. A rule-based method on polyphones phonetic notation on the 220 polyphones whose appearing frequency exceeds 99% is proposed. Firstly, all the polyphones 2.1 Classify the polyphones beforehand in a sentence are classified beforehand. Secondly, rules are Although the number of polyphones is large, the extracted based on eight features. Lastly, automatic phonetic appearing frequency is widely discrepant. The statistic notation is applied according to the rules. The paper puts forward results in our experiment show: the accumulative a new concept of prosodic functional part of speech in order to frequency of the former 100 polyphones exceeds 93%, improve the numerous and complicated grammatical information. and the accumulative frequency of the former 180 The examples and results of this method are shown at the end of polyphones exceeds 97%. The distributing chart of this paper. Compared with other approaches dealt with polyphones’ accumulative frequency is shown in the polyphones, the results show that this new method improves the chart 3.1. In order to improve the accuracy of the accuracy of phonetic notation on polyphones. grapheme-phoneme conversion more, we classify the Keyword: grapheme-phoneme conversion; polyphone; prosodic 700 polyphones in the GB_2312 Chinese-character word; prosodic functional part of speech; feature extracting; system into three kinds in our text-to-speech system 1. -

Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A
Article pubs.acs.org/joc Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A. Glover* and Adam A. Rosser Department of Chemistry, School of Science and Technology, University of New England, Armidale, NSW 2351, Australia *S Supporting Information ABSTRACT: Two independent computational methods have been used for determination of amide resonance stabilization and amidicities relative to N,N-dimethylacetamide for a wide range of acyclic and cyclic amides. The first method utilizes carbonyl substitution nitrogen atom replacement (COSNAR). The second, new approach involves determination of the difference in amide resonance between N,N-dimethylacetamide and the target amide using an isodesmic trans-amidation process and is calibrated relative to 1-aza-2-adamantanone with zero amidicity and N,N-dimethylace- tamide with 100% amidicity. Results indicate excellent coherence between the methods, which must be regarded as more reliable than a recently reported approach to amidicities based upon enthalpies of hydrogenation. Data for acyclic planar and twisted amides are predictable on the basis of the degrees of pyramidalization at nitrogen and twisting about the C−N bonds. Monocyclic lactams are predicted to have amidicities at least as high as N,N-dimethylacetamide, and the β-lactam system is planar with greater amide resonance than that of N,N- dimethylacetamide. Bicyclic penam/em and cepham/em scaffolds lose some amidicity in line with the degree of strain-induced pyramidalization at the bridgehead nitrogen and twist about the amide bond, but the most puckered penem system still retains substantial amidicity equivalent to 73% that of N,N-dimethylacetamide. ■ INTRODUCTION completed by a third resonance form, III, with no C−N pi Amide linkages are ubiquitous in proteins, peptides, and natural character and on account of positive polarity at carbon and the or synthetic molecules and in most of these they possess, electronegativity of nitrogen, must be destabilizing. -

The Romantext Format: a Flexible and Standard Method for Representing Roman Numeral Analyses
THE ROMANTEXT FORMAT: A FLEXIBLE AND STANDARD METHOD FOR REPRESENTING ROMAN NUMERAL ANALYSES Dmitri Tymoczko1 Mark Gotham2 Michael Scott Cuthbert3 Christopher Ariza4 1 Princeton University, NJ 2 Cornell University, NY 3 M.I.T., MA 4 Independent [email protected], [email protected], [email protected] ABSTRACT Absolute chord labels are performer-oriented in the sense that they specify which notes should be played, but Roman numeral analysis has been central to the West- do not provide any information about their function or ern musician’s toolkit since its emergence in the early meaning: thus one and the same symbol can represent nineteenth century: it is an extremely popular method for a tonic, dominant, or subdominant chord. Accordingly, recording subjective analytical decisions about the chords these labels obscure a good deal of musical structure: a and keys implied by a passage of music. Disagreements dominant-functioned G major chord (i.e. G major in the about these judgments have led to extensive theoretical de- local key of C) is considerably more likely to descend by bates and ongoing controversies. Such debates are exac- perfect fifth than a subdominant-functioned G major (i.e. G erbated by the absence of a public corpus of expert Ro- major in the key of D major). This sort of contextual in- man numeral analyses, and by the more fundamental lack formation is readily available to both trained and untrained of an agreed-upon, computer-readable syntax in which listeners, who are typically more sensitive to relative scale those analyses might be expressed. This paper specifies degrees than absolute pitches. -

Figured-Bass Notation
MU 182: Theory II R. Vigil FIGURED-BASS NOTATION General In common-practice tonal music, chords are generally understood in two different ways. On the one hand, they can be seen as triadic structures emanating from a generative root . In this system, a root-position triad is understood as the "ideal" or "original" form, and other forms are understood as inversions , where the root has been placed above one of the other chord tones. This approach emphasizes the structural similarity of chords that share a common root (a first- inversion C major triad and a root-position C major triad are both C major triads). This type of thinking is represented analytically in the practice of applying Roman numerals to various chords within a given key - all chords with allegiance to the same Roman numeral are understood to be related, regardless of inversion and voicing, texture, etc. On the other hand, chords can be understood as vertical arrangements of tones above a given bass . This system is not based on a judgment as to the primacy of any particular chordal arrangement over another. Rather, it is simply a descriptive mechanism, for identifying what notes are present in addition to the bass. In this regime, chords are described in terms of the simplest possible arrangement of those notes as intervals above the bass. The intervals are represented as Arabic numerals (figures), and the resulting nomenclatural system is known as figured bass . Terminological Distinctions Between Roman Numeral Versus Figured Bass Approaches When dealing with Roman numerals, everything is understood in relation to the root; therefore, the components of a triad are the root, the third, and the fifth. -

The Birth of Modern Chemistry
The Birth of Modern Chemistry 3rd semester project, Fall 2008 NIB Group nr.5, House 13.2 Thomas Allan Rayner & Aiga Mackevica Supervisor: Torben Brauner Abstract Alchemy was a science practiced for more than two millennia up till the end of 18th century when it was replaced by modern chemistry, which is practiced up till this very day. The purpose of this report is to look into this shift and investigate whether this shift can be classified as a paradigm shift according to the famous philosopher Thomas Kuhn, who came up with a theory on the structure of scientific revolutions. In order to come to draw any kind of conclusions, the report summarizes and defines the criteria for what constitutes a paradigm, crisis and paradigm shift, which are all important in order to investigate the manner in which a paradigm shift occurs. The criteria are applied to the historical development of modern chemistry and alchemy as well as the transition between the two sciences. As a result, alchemy and modern chemistry satisfy the requirements and can be viewed as two different paradigms in a Kuhnian sense. However, it is debatable whether the shift from alchemy to modern chemistry can be called a paradigm shift. 2 Acknowledgments We would like to thank Torben Brauner for his guidance throughout the project period as our supervisor. We would also like to thank our opposing group – Paula Melo Paulon Hansen, Stine Hesselholt Sloth and their supervisor Ole Andersen for their advice and critique for our project. 3 Table of Contents 1. Introduction ................................................................................................................................ 5 2. -

General Chemistry Course Description
Chemistry 110: General Chemistry Note: this is a representative syllabus for Chemistry 110. It is here for informational purposes. It is not intended to substitute for or replace the syllabus your instructor provides. Course Description: Introduction to the general principles of chemistry for students planning a professional career in chemistry, a related science, the health professions, or engineering. Stoichiometry, atomic structure, chemical bonding and geometry, thermochemistry, gases, types of chemical reactions, statistics. Weekly laboratory exercises emphasize quantitative techniques and complement the lecture material. Weekly discussion sessions focus on homework assignments and lecture material. Prerequisite: Mathematics 055 or equivalent. Previous high school chemistry not required. Taught: Annually, 5 semester credits. Course Components: Lecture, Recitation section, Laboratory Textbook: Steven S. Zumdahl and Susan A. Zumdahl, Chemistry, 5th Ed., Houghton Mifflin Company (New York, 2000). The text is available in the college bookstore. Lab Manual: Chemistry Department Faculty, Chemistry 110 Lab Manual, Fall, 2001 Edition, Kinkos. These may be purchased from Linda Noble, the Chemistry Secretary. Safety Goggles: These must be worn at all times in the laboratory. They can be purchased in the college bookstore. Calculator: Must have logs and exponential capability. Lab Notebook: This is a bound duplicate notebook with numbered pages. It can be purchased in the college bookstore Lecture Schedule 1.1-1.5 introduction, laboratory precision -

Course Syllabus
Course Syllabus Department: Science & Technology Date: January 2015 I. Course Prefix and Number: CHM 205 Course Name: Organic Chemistry I – Lecture Only Credit Hours and Contact Hours: 4 credit hours and 4 (3:0:1) contact hours Catalog Description including pre- and co-requisites: A systematic study of the chemistry of carbon compounds emphasizing reactions, mechanisms, and synthesis with a focus on functional groups, addition reactions to alkenes and alkynes, alcohols and ethers, stereochemistry, nomenclature, acid-base chemistry, reaction kinetics and thermodynamics. Completion of General Chemistry II or equivalent with a grade of C or better is prerequisite. II. Course Outcomes and Objectives Student Learning Outcomes: Upon completion of this course, the student will be able to: Demonstrate an understanding of basic principles of organic chemistry and how they relate to everyday experiences. Demonstrate problem solving and critical thinking skills Apply methods of scientific inquiry. Apply problem solving techniques to real-world problems. Demonstrate an understanding of the chemical environment and the role that organic molecules play in the natural and the synthetic world. Relationship to Academic Programs and Curriculum: This course is required for majors in chemistry, chemical engineering, biology, biotechnology, pharmacology, and other pre-professional programs. College Learning Outcomes Addressed by the Course: writing computer literacy oral communications ethics/values X reading citizenship mathematics global concerns X critical thinking information resources 1 III. Instructional Materials and Methods Types of Course Materials: A standard two-semester 200 level organic textbook and workbook. Methods of Instruction (e.g. Lecture and Seminar …): Three hours of lecture, with a one hour recitation period for individual as well as group learning activities such as case studies and guided learning activities. -

Notation Guide for Precalculus and Calculus Students
Notation Guide for Precalculus and Calculus Students Sean Raleigh Last modified: August 27, 2007 Contents 1 Introduction 5 2 Expression versus equation 7 3 Handwritten math versus typed math 9 3.1 Numerals . 9 3.2 Letters . 10 4 Use of calculators 11 5 General organizational principles 15 5.1 Legibility of work . 15 5.2 Flow of work . 16 5.3 Using English . 18 6 Precalculus 21 6.1 Multiplication and division . 21 6.2 Fractions . 23 6.3 Functions and variables . 27 6.4 Roots . 29 6.5 Exponents . 30 6.6 Inequalities . 32 6.7 Trigonometry . 35 6.8 Logarithms . 38 6.9 Inverse functions . 40 6.10 Order of functions . 42 7 Simplification of answers 43 7.1 Redundant notation . 44 7.2 Factoring and expanding . 45 7.3 Basic algebra . 46 7.4 Domain matching . 47 7.5 Using identities . 50 7.6 Log functions and exponential functions . 51 7.7 Trig functions and inverse trig functions . 53 1 8 Limits 55 8.1 Limit notation . 55 8.2 Infinite limits . 57 9 Derivatives 59 9.1 Derivative notation . 59 9.1.1 Lagrange’s notation . 59 9.1.2 Leibniz’s notation . 60 9.1.3 Euler’s notation . 62 9.1.4 Newton’s notation . 63 9.1.5 Other notation issues . 63 9.2 Chain rule . 65 10 Integrals 67 10.1 Integral notation . 67 10.2 Definite integrals . 69 10.3 Indefinite integrals . 71 10.4 Integration by substitution . 72 10.5 Improper integrals . 77 11 Sequences and series 79 11.1 Sequences . -
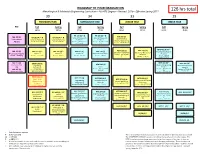
BS Metallurgical Engineering Curriculum Flowchart
ROADMAP TO YOUR GRADUATION Metallurgical & Materials Engineering Curriculum – BS MTE Degree – Revised 2016-- Effective Spring 2017 126 hrs total 30 34 33 29 FRESHMAN YEAR SOPHOMORE YEAR JUNIOR YEAR SENIOR YEAR Pre Fall SprIng Fall SprIng Fall SprIng Fall SprIng 16 hrs 14 hrs 17 hrs 17 hrs 16 hrs 17 hrs 14 hrs 15 hrs PH 105 (4) * N PH 106 (4) * N ECE 320 (3) MA 100 (3) CH 101 (4) * N CH 102 (4) * N General Physics with General Physics with Fundamentals of Intermediate General Chemistry 1 General Chemistry 2 Calculus 1 Calculus 2 Electrical Engineering Pre-reqs. = See catalog Pre-reqs. = CH 101 Pre-reqs. = MA 113 or 115 Pre-reqs. = PH 101 or 105 or Algebra Pre-reqs. = PH 106, MA 238 or 125 or 145 125 MTE 481 (4) W * AEM 250 (3) MTE 455 (4) MA 112 (3) MA 125 (4) * MA 126 (4) * MA 227 (4) MA 238 (3) Analytical Methods for Mechanics of Materials Mechanical Behavior of Precalculus Algebra Calculus 1 Calculus 2 Calculus 3 Differential Equations 1 Materials Pre-reqs. = MA 126, AEM Materials Pre-reqs. = See catalog Pre-reqs. = See catalog Pre-reqs. = MA 125 Pre-reqs. = MA 126 Pre-reqs. = MA 126 Pre-req. = MA 238 201 Pre-reqs. = AEM 250 Co-req. = MTE 441 # MA 113 (3) ENGR 103 (3) MTE 443 (3) MTE 445 (3) # AEM 201 (3) Materials Engineering Precalculus Trig Engineering Materials Engineering Statics Design 1 OR Foundations Design 2 Pre-reqs. = MA 125, PH 105, Pre-reqs. = MTE 362, 373, Pre-reqs. = MATH 125 or Pre-reqs. -

Professor of Chemistry
Bruce C. Gibb FRSC Professor of Chemistry Department of Chemistry Tulane University New Orleans, LA 70118, USA Tel: (504) 862 8136 E-mail: [email protected] Website: http://www.gibbgroup.org Twitter: @brucecgibb Research Interests: Aqueous supramolecular chemistry: understanding how molecules interact in water: from specific ion- pairing and the hydrophobic effect, to protein aggregation pertinent to neurodegenerative disorders. Our research has primarily focused on: 1) novel hosts designed to probe the hydrophobic, Hofmeister, and Reverse Hofmeister effects, and; 2) designing supramolecular capsules as yocto-liter reaction vessels and separators. Current efforts to probe the hydrophobic and Hofmeister effects include studies of the supramolecular properties of proteins. Professional Positions: Visiting Professor, Wuhan University of Science and Technology as a Chair Professor of Chutian Scholars Program (2015-2018) Professor of Chemistry, Tulane University, New Orleans, USA (2012-present). University Research Professor, University of New Orleans, USA (2007-2011). Professor of Chemistry, University of New Orleans, USA (2005-2007). Associate Professor of Chemistry, University of New Orleans, USA (2002-2005). Assistant Professor of Chemistry, University of New Orleans, USA, (1996-2002). Education: Postdoctoral Work Department of Chemistry, New York University. Synthesis of Carbonic Anhydrase (CA) mimics with Advisor: Prof. J. W. Canary, (1994-1996). Department of Chemistry, University of British Columbia, Canada De Novo Protein development. Advisor: Prof. J. C. Sherman (1993-1994). Ph.D. Robert Gordon’s University, Aberdeen, UK. Synthesis and Structural Examination of 3a,5-cyclo-5a- Androstane Steroids. Advisors: Dr. Philip J. Cox and Dr. Steven MacManus (1987-92) . B.Sc. with Honors in Physical Sciences Robert Gordon’s University, Aberdeen, UK.