Professor of Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -

Part III Project 2016/17 Professor Chris Hunter Physical Organic
Department of Chemistry: Part III Project 2016/17 Professor Chris Hunter Physical Organic Chemistry or Synthetic Supramolecular Chemistry EMAIL [email protected] Group web site http://www-hunter.ch.cam.ac.uk/ Contact details: I will be available to discuss projects on Monday 15th May (please email to make an appointment) The success of synthetic chemistry in the last century was built on the development of a set of design rules that allowed the quantitative prediction of reactivity and conformation based simply on chemical structure. The goal of our research is to establish a comparable set of rules that can be used for the design of non-covalent systems with equal reliability. Research projects are available in different areas and can be tailored to involve combinations of different techniques: organic synthesis; coordination chemistry; structural and thermodynamic characterisation of intermolecular complexes using NMR spectroscopy, mass spectrometry, X-ray crystallography; high-throughput physical organic chemistry; molecular design and molecular modelling. Physical Organic Chemistry: Quantitative Non-Covalent Chemistry Synthetic supramolecular systems are ideally suited for the systematic study and quantitative determination of the thermodynamic properties of non-covalent interactions. We are developing new experimental methods for quantifying the relative contributions of different factors that influence the behaviour of complex systems. By characterising the relationship between chemical structure and thermodynamic properties, we aim to develop rules of thumb (and software) for predicting the properties of molecular systems based on a quantitative fundamental understanding of non-covalent interactions. This project will involve some synthetic chemistry, but the focus will be on quantitative physical measurements using a variety of spectroscopic techniques and instrumentation as well as mathematical model building. -

Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A
Article pubs.acs.org/joc Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A. Glover* and Adam A. Rosser Department of Chemistry, School of Science and Technology, University of New England, Armidale, NSW 2351, Australia *S Supporting Information ABSTRACT: Two independent computational methods have been used for determination of amide resonance stabilization and amidicities relative to N,N-dimethylacetamide for a wide range of acyclic and cyclic amides. The first method utilizes carbonyl substitution nitrogen atom replacement (COSNAR). The second, new approach involves determination of the difference in amide resonance between N,N-dimethylacetamide and the target amide using an isodesmic trans-amidation process and is calibrated relative to 1-aza-2-adamantanone with zero amidicity and N,N-dimethylace- tamide with 100% amidicity. Results indicate excellent coherence between the methods, which must be regarded as more reliable than a recently reported approach to amidicities based upon enthalpies of hydrogenation. Data for acyclic planar and twisted amides are predictable on the basis of the degrees of pyramidalization at nitrogen and twisting about the C−N bonds. Monocyclic lactams are predicted to have amidicities at least as high as N,N-dimethylacetamide, and the β-lactam system is planar with greater amide resonance than that of N,N- dimethylacetamide. Bicyclic penam/em and cepham/em scaffolds lose some amidicity in line with the degree of strain-induced pyramidalization at the bridgehead nitrogen and twist about the amide bond, but the most puckered penem system still retains substantial amidicity equivalent to 73% that of N,N-dimethylacetamide. ■ INTRODUCTION completed by a third resonance form, III, with no C−N pi Amide linkages are ubiquitous in proteins, peptides, and natural character and on account of positive polarity at carbon and the or synthetic molecules and in most of these they possess, electronegativity of nitrogen, must be destabilizing. -

Read the Book of Abstracts
2o21 ONLINE May 10- 12 2021 International Symposium on SupraBiomolecular Systems 2021 Table of Contents TBD 1 Prof. Andreas Herrmann TBD 2 Prof. Rachel O’Reilly Tackling challenges in nanomedicine with responsive supramolecular polymers and advanced mi- croscopy 3 Dr. Silvia Pujals, Mr. Edgar Fuentes, Dr. Lorenzo Albertazzi Water Soluble Nanotubular Architectures from Amphiphilic Dinucleobases 4 Dr. Fatima Aparicio, Ms. Paula Blue Chamorro, Dr. Raquel Chamorro, Prof. David Gonzalez-Rodriguez Glyconucleolipids as new drug delivery systems for Parkinson’s disease treatment 5 Mr. Anthony Cunha, Dr. Alexandra Gaubert, Prof. Philippe Barthélémy, Dr. Benjamin Dehay, Dr. Laurent Latxague Membraneless compartments based on intrinsically disordered proteins: from biology towards new pro- tein materials 6 Prof. Paolo Arosio Low Molecular Weight Oleogel formation via unique keto-enol-type nucleolipid supramolecular assembly 8 Mr. Arthur KLUFTS-EDEL, Ms. Bérangère Dessane, Dr. Aladin Hamoud, Dr. Geoffrey Prévot, Dr. Antoine Lo- quet, Dr. Brice Kauffmann, Prof. Philippe Barthélémy, Prof. Sylvie Crauste-Manciet, Dr. Valérie Desvergnes Multi-responsive supramolecular fibers from peptide-based amphiphiles 9 Mr. Edgar Fuentes, Dr. Marieke Gerth, Dr. Jose Augusto Berrocal, Dr. Carlo Matera, Prof. Pau Gorostiza, Prof. Ilja Voets, Dr. Silvia Pujals, Dr. Lorenzo Albertazzi Electrostatic Protein Assemblies Towards Biohybrid Photoactive Materials 10 Dr. Eduardo Anaya-Plaza, Prof. Mauri Kostiainen Exploring Polyoxometalates as Non-destructive Staining Agents for Contrast-Enhanced Microfocus Com- puted Tomography of Biological Tissues 11 Ms. Sarah Vangrunderbeeck, Mr. Sébastien De Bournonville, Mrs. Hong Giang T. Ly, Prof. Wim De Borggraeve, Prof. Tatjana Parac-Vogt, Prof. Greet Kerckhofs Hydrogels with Photo-Switchable Stiffness: A Tool to Mimic Extra Cellular Matrix 13 Prof. -

Supramolecular Catalysts Green Chemistry
139 7 Supramolecular Catalysis as a Tool for Green Chemistry Courtney J. Hastings 7.1 Introduction Catalysis is central to advancing green chemistry in the area of synthetic chemis- try [1,2]. Beyond replacing stoichiometric reagents, catalysts have the potential to streamline multistep synthesis by enabling new bond-forming processes to shorten synthetic sequences and achieve better step economy [3,4]. Supra- molecular catalysis and the application of supramolecular concepts to catalytic reactions is emerging as a valuable tool for improving catalytic reactions for syn- thetic chemistry. Supramolecular catalysis can enable aqueous reaction condi- tions, improve reactions selectivity, improve catalyst lifetime, and enable tandem reactions, all of which can have positive impacts on the cost, waste, and energy associated with a reaction. The field of supramolecular chemistry concerns the design of molecular enti- ties that are defined by reversible, noncovalent interactions. While each supra- molecular interaction is quite weak individually, the effect of many such interactions working in concert can produce strongly associated and structurally well-defined molecular species [5–7]. Such additive effects are responsible for the spectacular structural complexity found in biomacromolecules such as pro- teins. Efforts to characterize these interactions have provided chemists with a “toolbox” of reliable methods to program the association between two or more molecules to form a single complexed species. Thus, supramolecular chemistry represents a complementary approach toward molecular construction, and one that offers certain advantages over covalent chemistry [5–8]. Like supramolecular interactions, host–guest binding relies on manifold non- covalent interactions, with the added requirement that the host possess an inte- rior cavity that is complementary in size and shape to the guest molecule [9–11]. -

The Birth of Modern Chemistry
The Birth of Modern Chemistry 3rd semester project, Fall 2008 NIB Group nr.5, House 13.2 Thomas Allan Rayner & Aiga Mackevica Supervisor: Torben Brauner Abstract Alchemy was a science practiced for more than two millennia up till the end of 18th century when it was replaced by modern chemistry, which is practiced up till this very day. The purpose of this report is to look into this shift and investigate whether this shift can be classified as a paradigm shift according to the famous philosopher Thomas Kuhn, who came up with a theory on the structure of scientific revolutions. In order to come to draw any kind of conclusions, the report summarizes and defines the criteria for what constitutes a paradigm, crisis and paradigm shift, which are all important in order to investigate the manner in which a paradigm shift occurs. The criteria are applied to the historical development of modern chemistry and alchemy as well as the transition between the two sciences. As a result, alchemy and modern chemistry satisfy the requirements and can be viewed as two different paradigms in a Kuhnian sense. However, it is debatable whether the shift from alchemy to modern chemistry can be called a paradigm shift. 2 Acknowledgments We would like to thank Torben Brauner for his guidance throughout the project period as our supervisor. We would also like to thank our opposing group – Paula Melo Paulon Hansen, Stine Hesselholt Sloth and their supervisor Ole Andersen for their advice and critique for our project. 3 Table of Contents 1. Introduction ................................................................................................................................ 5 2. -

General Chemistry Course Description
Chemistry 110: General Chemistry Note: this is a representative syllabus for Chemistry 110. It is here for informational purposes. It is not intended to substitute for or replace the syllabus your instructor provides. Course Description: Introduction to the general principles of chemistry for students planning a professional career in chemistry, a related science, the health professions, or engineering. Stoichiometry, atomic structure, chemical bonding and geometry, thermochemistry, gases, types of chemical reactions, statistics. Weekly laboratory exercises emphasize quantitative techniques and complement the lecture material. Weekly discussion sessions focus on homework assignments and lecture material. Prerequisite: Mathematics 055 or equivalent. Previous high school chemistry not required. Taught: Annually, 5 semester credits. Course Components: Lecture, Recitation section, Laboratory Textbook: Steven S. Zumdahl and Susan A. Zumdahl, Chemistry, 5th Ed., Houghton Mifflin Company (New York, 2000). The text is available in the college bookstore. Lab Manual: Chemistry Department Faculty, Chemistry 110 Lab Manual, Fall, 2001 Edition, Kinkos. These may be purchased from Linda Noble, the Chemistry Secretary. Safety Goggles: These must be worn at all times in the laboratory. They can be purchased in the college bookstore. Calculator: Must have logs and exponential capability. Lab Notebook: This is a bound duplicate notebook with numbered pages. It can be purchased in the college bookstore Lecture Schedule 1.1-1.5 introduction, laboratory precision -

Course Syllabus
Course Syllabus Department: Science & Technology Date: January 2015 I. Course Prefix and Number: CHM 205 Course Name: Organic Chemistry I – Lecture Only Credit Hours and Contact Hours: 4 credit hours and 4 (3:0:1) contact hours Catalog Description including pre- and co-requisites: A systematic study of the chemistry of carbon compounds emphasizing reactions, mechanisms, and synthesis with a focus on functional groups, addition reactions to alkenes and alkynes, alcohols and ethers, stereochemistry, nomenclature, acid-base chemistry, reaction kinetics and thermodynamics. Completion of General Chemistry II or equivalent with a grade of C or better is prerequisite. II. Course Outcomes and Objectives Student Learning Outcomes: Upon completion of this course, the student will be able to: Demonstrate an understanding of basic principles of organic chemistry and how they relate to everyday experiences. Demonstrate problem solving and critical thinking skills Apply methods of scientific inquiry. Apply problem solving techniques to real-world problems. Demonstrate an understanding of the chemical environment and the role that organic molecules play in the natural and the synthetic world. Relationship to Academic Programs and Curriculum: This course is required for majors in chemistry, chemical engineering, biology, biotechnology, pharmacology, and other pre-professional programs. College Learning Outcomes Addressed by the Course: writing computer literacy oral communications ethics/values X reading citizenship mathematics global concerns X critical thinking information resources 1 III. Instructional Materials and Methods Types of Course Materials: A standard two-semester 200 level organic textbook and workbook. Methods of Instruction (e.g. Lecture and Seminar …): Three hours of lecture, with a one hour recitation period for individual as well as group learning activities such as case studies and guided learning activities. -

Synthesis of Organometallic PNA Oligomers by Click Chemistry
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008 1 Supporting Information: Synthesis of organometallic PNA oligomers by click chemistry Gilles Gasser, Nina Hüsken, S. David Köster and Nils Metzler-Nolte* Department of Inorganic Chemistry I – Bioinorganic Chemistry, Faculty of Chemistry and Biochemistry, Ruhr-University Bochum Universitätsstrasse 150; 44801 Bochum, Germany Fax: +49 (0)234 – 32 14378; E-Mail: [email protected] Table of contents I. Experimental Section a) Materials 2 b) Instrumentation and methods 2 c) Peptide Nucleic Acid synthesis 2 d) Synthesis, characterisation and NMR spectra of Fmoc-1-OtBu, Fmoc-1-OH, Fmoc-2-OtBu and 3 3 e) Characterisation of PNA1-4 10 f) Synthesis and characterisation of Fc-PNA1-3 and Fc2-PNA4 10 II. MALDI-TOF Spectra a) Figure 5: MALDI-TOF mass spectrum of Fc-PNA1 11 b) Figure 6: MALDI-TOF mass spectrum of Fc-PNA2 12 c) Figure 7: MALDI-TOF mass spectrum of Fc-PNA3 12 d) Figure 8: MALDI-TOF mass spectrum of Fc2-PNA4 13 III. References 13 Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008 2 I. Experimental Section a) Materials. All chemicals were of reagent grade quality or better, obtained from commercial suppliers and used without further purification. Solvents were used as received or dried over 4 Å molecular sieves. b) Instrumentation and methods. 1H and 13C NMR spectra were recorded in deuterated solvents on a Bruker DRX 400 spectrometer at 30°C. The chemical shifts, δ, are reported in ppm (parts per million). -
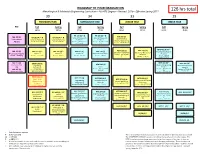
BS Metallurgical Engineering Curriculum Flowchart
ROADMAP TO YOUR GRADUATION Metallurgical & Materials Engineering Curriculum – BS MTE Degree – Revised 2016-- Effective Spring 2017 126 hrs total 30 34 33 29 FRESHMAN YEAR SOPHOMORE YEAR JUNIOR YEAR SENIOR YEAR Pre Fall SprIng Fall SprIng Fall SprIng Fall SprIng 16 hrs 14 hrs 17 hrs 17 hrs 16 hrs 17 hrs 14 hrs 15 hrs PH 105 (4) * N PH 106 (4) * N ECE 320 (3) MA 100 (3) CH 101 (4) * N CH 102 (4) * N General Physics with General Physics with Fundamentals of Intermediate General Chemistry 1 General Chemistry 2 Calculus 1 Calculus 2 Electrical Engineering Pre-reqs. = See catalog Pre-reqs. = CH 101 Pre-reqs. = MA 113 or 115 Pre-reqs. = PH 101 or 105 or Algebra Pre-reqs. = PH 106, MA 238 or 125 or 145 125 MTE 481 (4) W * AEM 250 (3) MTE 455 (4) MA 112 (3) MA 125 (4) * MA 126 (4) * MA 227 (4) MA 238 (3) Analytical Methods for Mechanics of Materials Mechanical Behavior of Precalculus Algebra Calculus 1 Calculus 2 Calculus 3 Differential Equations 1 Materials Pre-reqs. = MA 126, AEM Materials Pre-reqs. = See catalog Pre-reqs. = See catalog Pre-reqs. = MA 125 Pre-reqs. = MA 126 Pre-reqs. = MA 126 Pre-req. = MA 238 201 Pre-reqs. = AEM 250 Co-req. = MTE 441 # MA 113 (3) ENGR 103 (3) MTE 443 (3) MTE 445 (3) # AEM 201 (3) Materials Engineering Precalculus Trig Engineering Materials Engineering Statics Design 1 OR Foundations Design 2 Pre-reqs. = MA 125, PH 105, Pre-reqs. = MTE 362, 373, Pre-reqs. = MATH 125 or Pre-reqs. -

Molecular Electronic Devices Based on Single-Walled Carbon Nanotube Electrodes Alina K
Subscriber access provided by Columbia Univ Libraries Article Molecular Electronic Devices Based on Single-Walled Carbon Nanotube Electrodes Alina K. Feldman, Michael L. Steigerwald, Xuefeng Guo, and Colin Nuckolls Acc. Chem. Res., 2008, 41 (12), 1731-1741• DOI: 10.1021/ar8000266 • Publication Date (Web): 18 September 2008 Downloaded from http://pubs.acs.org on May 14, 2009 More About This Article Additional resources and features associated with this article are available within the HTML version: • Supporting Information • Access to high resolution figures • Links to articles and content related to this article • Copyright permission to reproduce figures and/or text from this article Accounts of Chemical Research is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036 Molecular Electronic Devices Based on Single-Walled Carbon Nanotube Electrodes ALINA K. FELDMAN,† MICHAEL L. STEIGERWALD,† XUEFENG GUO,*,‡ AND COLIN NUCKOLLS*,† †Department of Chemistry and the Columbia University Center for Electronics of Molecular Nanostructures, Columbia University, New York, New York 10027, ‡Centre for Nanochemistry (CNC), Beijing National Laboratory for Molecular Sciences (BNLMS), State Key Laboratory for Structural Chemistry of Unstable and Stable Species, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, P. R. China RECEIVED ON JANUARY 28, 2008 CON SPECTUS s the top-down fabrication techniques for silicon-based Aelectronic materials have reached the scale of molecu- lar lengths, researchers have been investigating nanostruc- tured materials to build electronics from individual molecules. Researchers have directed extensive experimental and the- oretical efforts toward building functional optoelectronic devices using individual organic molecules and fabricating metal-molecule junctions. Although this method has many advantages, its limitations lead to large disagreement between experimental and theoretical results. -

Comparative Analysis of Click Chemistry Mediated Activity-Based Protein Profiling in Cell Lysates
Molecules 2013, 18, 12599-12608; doi:10.3390/molecules181012599 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Communication Comparative Analysis of Click Chemistry Mediated Activity-Based Protein Profiling in Cell Lysates Yinliang Yang, Xiaomeng Yang and Steven H. L. Verhelst * Lehrstuhl für Chemie der Biopolymere, Technische Universität München, Weihenstephaner Berg 3, 85354 Freising, Germany; E-Mails: [email protected] (Y.Y.); [email protected] (X.Y.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-8161-713505; Fax: +49-8161-714404. Received: 26 June 2013; in revised form: 7 August 2013 / Accepted: 26 September 2013 / Published: 11 October 2013 Abstract: Activity-based protein profiling uses chemical probes that covalently attach to active enzyme targets. Probes with conventional tags have disadvantages, such as limited cell permeability or steric hindrance around the reactive group. A tandem labeling strategy with click chemistry is now widely used to study enzyme targets in situ and in vivo. Herein, the probes are reacted in live cells, whereas the ensuing detection by click chemistry takes place in cell lysates. We here make a comparison of the efficiency of the activity-based tandem labeling strategy by using Cu(I)-catalyzed and strain-promoted click chemistry, different ligands and different lysis conditions. Keywords: activity-based probes; cathepsins; click chemistry; proteases; protein modification 1. Introduction Within chemical biology, site specific protein modification by covalent small molecule probes is a powerful and often used technique to interrogate biomolecular interactions and protein function. The introduction of covalent small molecule probes can be based on different types of chemistries: probes may be incorporated by the use of exogenous or endogenous enzymes [1,2], or they can contain an intrinsic reactivity such as an electrophile or photocrosslinker that by itself forms a covalent bond to the target proteins [3–5].