Sweat Gland Myoepithelial Cell Differentiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

The Distribution of Sweat Glands Over the Human Body Has So Far Been In
NOTES ON THE VERTICAL DISTRIBUTION OF THE HUMAN SWEAT GLANDS SHUNZO TAKAGI AND KO TOBARU* Institute of Physiology, School of Medicine, University of Nagoya•õ The distribution of sweat glands over the human body has so far been in- vestigated in the dimension of area, and we have no general idea how deep they are distributed in the skin. In 1943, Kuno and his collaborators (3) expressed the opinion that chloride would be accumulated in the skin during the activity of sweat glands. The truth of this assumption has been confirmed with more certainty by Yoshimura and Chihaya (unpublished), who measured the chloride content in the skin tissue by means of Ag-AgCl electrodes. The chloride may presumably be accumulated in the immediate neighbourhood of the glomeruli of sweat glands, or more diffusely in the layers of skin tissues where the glomeruli are situated. For consideration of the amount of the accumulated chloride, the total volume of these skin layers, which can be estimated by the vertical distribution of the sweat-gland glomeruli, seems to be useful. The fol- lowing investigation was therefore performed. MATERIALS AND METHOD Skin samples of 33 regions of the body, as specified in table 1, were taken from the corpse of a Japanese male of 30 years old, who died an accidental death. The samples were fixed in 10 per cent formalin, embedded in celloidin and cut into sections 15 micra thick. The sections were stained with Delafield's hematoxylin and eosin. Observations of sweat glands were made with 2-3 pieces of the skin about 100 sq. -

Modelling Breast Epithelial-Endothelial Interaction in Three-Dimensional Cell Culture
Modelling breast epithelial-endothelial interaction in three-dimensional cell culture A thesis submitted for the degree of Master of Science Sævar Ingþórsson Department of Medicine University of Iceland Instructors and Masters Project Committee: Þórarinn Guðjónsson, Ph.D Magnús Karl Magnússon, MD Kristján Leósson, Ph.D Reykjavik, Iceland September 2008 Samspil æðaþels og eðlilegs og illkynja þekjuvefjar úr brjóstkirtli í þrívíðri frumurækt Ritgerð til meistaragráðu Sævar Ingþórsson Háskóli Íslands Læknadeild Leiðbeinendur og meistaranámsnefnd: Þórarinn Guðjónsson, Ph.D Magnús Karl Magnússon, MD Kristján Leósson, Ph.D Reykjavík, September 2008 Ágrip Brjóstkirtillinn samanstendur af tveimur megingerðum þekjuvefsfruma, kirtilþekju- og vöðvaþekjufrumum. Saman mynda þessar frumugerðir hina greinóttu formgerð brjóstkirtilsins. Kirtilvefurinn er umlukinn æðaríkum stoðvef sem inniheldur margar mismunandi frumugerðir, þ.m.t. bandvefsfrumur og æðaþelsfrumur. Þroskun og sérhæfing kirtilsins er mjög háð samskiptum hans við millifrumuefni brjóstsins og frumur stoðvefjarins. Mest áhersla hefur verið lögð á rannsóknir á bandvefsfrumum í þessu tilliti, en minni athygli beint að æðaþelsfrumum, sem voru lengi taldar gegna því hlutverki einu að miðla súrefni og næringu um líkamann. Á síðustu árum hefur verið sýnt fram á að nýmyndun æða í krabbameinsæxlum spili stórt hlutverk í framþróun æxlisvaxtar og hefur það verið tengt slæmum horfum. Nýlegar rannsóknir hafa sýnt fram á mikilvægt hlutverk æðaþels í þroskun og sérhæfingu ýmissa líffæra, til dæmis í heila, lifur og beinmerg sem og í framþróun krabbameins. Nýleg þekking bendir einnig til mikilvægra áhrifa æðaþels á þroskun eðlilegs og illkynja brjóstvefjar. Markmið verkefnisins er að kanna áhrif brjóstaæðaþels á eðlilegar og illkynja brjóstaþekjufrumulínur og nota til þess þrívíð ræktunarlíkön sem þróuð voru á rannsóknastofunni, sem og að endurbæta þessi líkön til frekari rannsókna á samskiptum æðaþels og þekjufruma. -

Periodic Acid-Schiff Positive Material Accumulating Within the Lumen of Eccrine Sweat Glands*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector PERIODIC ACID-SCHIFF POSITIVE MATERIAL ACCUMULATING WITHIN THE LUMEN OF ECCRINE SWEAT GLANDS* GEORGE W. HAMBRICK, JR., M.D. The purpose of this paper is to record findings with regard to diastase-resistant, periodic acid-Schiff positive material in the lumen of eccrine ducts and glands of two individuals. This material has two possible sources in the normal eccrine sweat gland, namely, the cuticle lining of the eccrine duct and the cells of the secretory tubule. Holyoke and Lobits (3) studying 35 normal skin biopsies re- B pp HI D FIG. 1. A. Vertical section through skin of the control area showing dilatation of an eccrine sweat duct with eosinophilic material in the lumen. H. and E., X178. B. Same find- ings as in A above from an area treated daily with 3 per cent hexachloronaphthalene in acetone for one week, Xl7S. C. Section through dermal parts of eccrine duct containing a cast. H. and E., X355. D. Section through dermal eccrine duct containing periodic acid- Schiff positive material in the lumen, X660. *Fromthe Department of Dermatology (Donald M. Pillsbury, M.D., Director), School of Medicine, University of Pennsylvania, Philadelphia 4, Pennsylvania. This study was supported by U. S. Army grant DA-49-007-MD-154. Received for publication March 29, 1957. 213 214 THF JOURNAL OF INVESTIGATIVE DERMATOLOGY ported the presence of material in the lumen of the eccrine duct and gland; they classified the material as amorphous, cast, cellular or bacterial. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Sweat Glands • Oil Glands • Mammary Glands
Chapter 4 The Integumentary System Lecture Presentation by Steven Bassett Southeast Community College © 2015 Pearson Education, Inc. Introduction • The integumentary system is composed of: • Skin • Hair • Nails • Sweat glands • Oil glands • Mammary glands © 2015 Pearson Education, Inc. Introduction • The skin is the most visible organ of the body • Clinicians can tell a lot about the overall health of the body by examining the skin • Skin helps protect from the environment • Skin helps to regulate body temperature © 2015 Pearson Education, Inc. Integumentary Structure and Function • Cutaneous Membrane • Epidermis • Dermis • Accessory Structures • Hair follicles • Exocrine glands • Nails © 2015 Pearson Education, Inc. Figure 4.1 Functional Organization of the Integumentary System Integumentary System FUNCTIONS • Physical protection from • Synthesis and storage • Coordination of immune • Sensory information • Excretion environmental hazards of lipid reserves response to pathogens • Synthesis of vitamin D3 • Thermoregulation and cancers in skin Cutaneous Membrane Accessory Structures Epidermis Dermis Hair Follicles Exocrine Glands Nails • Protects dermis from Papillary Layer Reticular Layer • Produce hairs that • Assist in • Protect and trauma, chemicals protect skull thermoregulation support tips • Nourishes and • Restricts spread of • Controls skin permeability, • Produce hairs that • Excrete wastes of fingers and supports pathogens prevents water loss provide delicate • Lubricate toes epidermis penetrating epidermis • Prevents entry of -

Histological Variations in Myoepithelial Cells and Arrectores Pilorum Muscles Among Caudal, Metatarsal and Preorbital Glands In
NOTE Anatomy Histological Variations in Myoepithelial Cells and Arrectores Pilorum Muscles among Caudal, Metatarsal and Preorbital Glands in Hokkaido Sika Deer (Cervus nippon yesoensis Heude, 1884) Nobuo OZAKI1), Masatsugu SUZUKI1)* and Noriyuki OHTAISHI1) 1)Laboratory of Wildlife Biology, The Graduate School of Veterinary Medicine, Hokkaido University, Kita-ku, Sapporo 060–0818, Japan (Received 1 April 2003/Accepted 15 October 2003) ABSTRACT. The morphological characteristics of myoepithelial cells and arrectores pilorum muscles were investigated in caudal, metatarsal and preorbital glands of Hokkaido sika deer (Cervus nippon yesoensis Heude, 1884) using immunohistochemistry for α-smooth muscle actin. In the metatarsal, preorbital and general skin glands, myoepithelial cell layers continuously embraced the secretory epithelium, while in the caudal gland, discontinuous myoepithelial cell rows surrounded the apocrine tubules. There was a trend that the widths of the myoepithelial cells of the caudal and preorbital glands appeared to be thinner than those of the metatarsal and general skin glands. In the metatarsal gland, the arrectores pilorum muscles were highly developed and considerably larger than those in other skin glands. KEY WORDS: arrectores pilorum muscle, myoepithelial cell, sika deer. J. Vet. Med. Sci. 66(3): 283–285, 2004 The specialized skin glands in the cervid species include were incubated for 15 min at room temperature with a bioti- forehead, preorbital, tarsal, metatarsal, interdigital and cau- nylated rabbit antibody against mouse IgG, IgA and IgM dal glands [15], most of which contain both apocrine and (Nichirei). Then they were rinsed three times (10 min each) sebaceous glandular elements [1, 7, 12]. In Hokkaido sika in PBS and incubated for 10 min at room temperature in a deer (Cervus nippon yesoensis Heude, 1884), the existence streptavidin-biotin peroxidase complex (Nichirei). -

The Mammary Myoepithelial Cell MEJDI MOUMEN, AURÉLIE CHICHE, STÉPHANIE CAGNET, VALÉRIE PETIT, KARINE RAYMOND, MARISA M
Int. J. Dev. Biol. 55: 763-771 doi: 10.1387/ijdb.113385mm www.intjdevbiol.com The mammary myoepithelial cell MEJDI MOUMEN, AURÉLIE CHICHE, STÉPHANIE CAGNET, VALÉRIE PETIT, KARINE RAYMOND, MARISA M. FARALDO, MARIE-ANGE DEUGNIER and MARINA A. GLUKHOVA* CNRS, UMR 144 Institut Curie "Compartimentation et Dynamique Cellulaires", Paris, France ABSTRACT Over the last few years, the discovery of basal-type mammary carcinomas and the association of the regenerative potential of the mammary epithelium with the basal myoepithelial cell population have attracted considerable attention to this second major mammary lineage. Howe- ver, many questions concerning the role of basal myoepithelial cells in mammary morphogenesis, functional differentiation and disease remain unanswered. Here, we discuss the mechanisms that control the myoepithelial cell differentiation essential for their contractile function, summarize new data concerning the roles played by cell-extracellular matrix (ECM), intercellular and paracrine interactions in the regulation of various aspects of the mammary basal myoepithelial cell functional activity. Finally, we analyze the contribution of the basal myoepithelial cells to the regenerative potential of the mammary epithelium and tumorigenesis. KEY WORDS: differentiation, extracellular matrix, intercellular signaling, stem cells, tumorigenesis Introduction in permanent reciprocal interactions with the connective tissue surrounding the mammary epithelium. Functionally differentiated mammary gland consists of secretory The myoepithelium -

HYPERHIDROSIS 101: Understanding Excessive Sweat
HYPERHIDROSIS 101: Understanding Excessive Sweat Hyperhidrosis is a medical condition in which excessive sweating occurs beyond what is needed to maintain normal body temperature.1,2 Excessive sweating can occur in the hands, feet, underarms or face, and it often interferes with everyday activities.1 Understanding Sweat Sweat glands are small tubular structures in the skin that secrete sweat onto the skin via a duct.3 Eccrine sweat glands are distributed throughout almost the entire human body and they secrete directly onto the surface of the skin.3 Apocrine sweat glands are ten times larger than eccrine sweat glands.3 They are localized in the axilla (underarms) and perianal areas.3 Rather than directly opening onto the skin surface, these glands secrete sweat into the pilary canal of the hair follicle.3 What is Hyperhidrosis? We all sweat. It’s the body’s way of cooling itself and preventing 1 ourselves from overheating. People living with hyperhidrosis, What Causes Us to Sweat? however, sweat when the body doesn’t necessarily need cooling.1 Their sweat is excessive, often visible to others and usually occurs The main reason we sweat is to control without physical exertion or extreme heat. our body temperature.2 There are two different types of hyperhidrosis - primary (also known as focal Here’s how it works: Sensors in our skin can detect changes in temperature hyperhidrosis) and secondary hyperhidrosis.6 Primary hyperhidrosis often begins and relay signals to our brain when we in childhood or adolescence and affects localized areas of the body, including the exercise or when it is hot outside.4 In hands, feet, underarms or face.6 The condition may be inherited and many members turn, our brain signals the sweat glands 7 of the same family may suffer from hyperhidrosis. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -
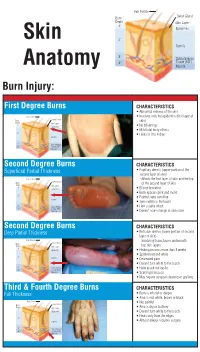
Identifying the Degree of a Burn Poster
Hair Folicle Sweat Gland Burn Depth Skin Layer 1° Epidermis Skin 2° Dermis 3° Subcutaneous 4° Tissue (FAT) Anatomy Muscle Burn Injury: First Degree Burns CHARACTERISTICS • Abnormal redness of the skin Hair Folicle • Involves only the epidermis (first layer of Sweat Gland Burn skin) Depth Skin Layer 1° Epidermis • No blistering • Mild total body effects 2° • Heals in 3 to 4 days Dermis 3° Subcutaneous 4° Tissue (FAT) Muscle Second Degree Burns CHARACTERISTICS Superficial Partial Thickness • Papillary dermis (upper portion of the second layer of skin) Hair Folicle – Affects the first layer of skin and the top Sweat Gland Burn of the second layer of skin Depth Skin Layer 1° Epidermis • Blister formation • Burn appears pink and moist 2° • Painful, very sensitive Dermis • Turns white to the touch 3° Subcutaneous • Hair usually intact 4° Tissue (FAT) Muscle • Doesn’t scar–change in skin color Second Degree Burns CHARACTERISTICS Deep Partial Thickness • Reticular dermis (lower portion of second layer of skin) Hair Folicle – Involving tissue layers underneath Sweat Gland Burn Depth Skin Layer top skin layers 1° Epidermis • Healing process more than 3 weeks • Spotted red and white 2° Dermis • Decreased pain • Doesn’t turn white to the touch 3° Subcutaneous • Hairs pluck out easily 4° Tissue (FAT) Muscle • Scarring increases • May require surgical cleaning or grafting Third & Fourth Degree Burns CHARACTERISTICS Full Thickness • Burn is into fat or deeper • Area is red, white, brown or black Hair Folicle • Not painful Sweat Gland Burn Depth Skin Layer • Area is dry or leathery 1° Epidermis • Doesn’t turn white to the touch • Heals only from the edges 2° Dermis • Almost always requires surgery 3° Subcutaneous 4° Tissue (FAT) Muscle. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization