Human Sweat Gland Myoepithelial Cells Express a Unique Set Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development and Maintenance of Epidermal Stem Cells in Skin Adnexa
International Journal of Molecular Sciences Review Development and Maintenance of Epidermal Stem Cells in Skin Adnexa Jaroslav Mokry * and Rishikaysh Pisal Medical Faculty, Charles University, 500 03 Hradec Kralove, Czech Republic; [email protected] * Correspondence: [email protected] Received: 30 October 2020; Accepted: 18 December 2020; Published: 20 December 2020 Abstract: The skin surface is modified by numerous appendages. These structures arise from epithelial stem cells (SCs) through the induction of epidermal placodes as a result of local signalling interplay with mesenchymal cells based on the Wnt–(Dkk4)–Eda–Shh cascade. Slight modifications of the cascade, with the participation of antagonistic signalling, decide whether multipotent epidermal SCs develop in interfollicular epidermis, scales, hair/feather follicles, nails or skin glands. This review describes the roles of epidermal SCs in the development of skin adnexa and interfollicular epidermis, as well as their maintenance. Each skin structure arises from distinct pools of epidermal SCs that are harboured in specific but different niches that control SC behaviour. Such relationships explain differences in marker and gene expression patterns between particular SC subsets. The activity of well-compartmentalized epidermal SCs is orchestrated with that of other skin cells not only along the hair cycle but also in the course of skin regeneration following injury. This review highlights several membrane markers, cytoplasmic proteins and transcription factors associated with epidermal SCs. Keywords: stem cell; epidermal placode; skin adnexa; signalling; hair pigmentation; markers; keratins 1. Epidermal Stem Cells as Units of Development 1.1. Development of the Epidermis and Placode Formation The embryonic skin at very early stages of development is covered by a surface ectoderm that is a precursor to the epidermis and its multiple derivatives. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

The Distribution of Sweat Glands Over the Human Body Has So Far Been In
NOTES ON THE VERTICAL DISTRIBUTION OF THE HUMAN SWEAT GLANDS SHUNZO TAKAGI AND KO TOBARU* Institute of Physiology, School of Medicine, University of Nagoya•õ The distribution of sweat glands over the human body has so far been in- vestigated in the dimension of area, and we have no general idea how deep they are distributed in the skin. In 1943, Kuno and his collaborators (3) expressed the opinion that chloride would be accumulated in the skin during the activity of sweat glands. The truth of this assumption has been confirmed with more certainty by Yoshimura and Chihaya (unpublished), who measured the chloride content in the skin tissue by means of Ag-AgCl electrodes. The chloride may presumably be accumulated in the immediate neighbourhood of the glomeruli of sweat glands, or more diffusely in the layers of skin tissues where the glomeruli are situated. For consideration of the amount of the accumulated chloride, the total volume of these skin layers, which can be estimated by the vertical distribution of the sweat-gland glomeruli, seems to be useful. The fol- lowing investigation was therefore performed. MATERIALS AND METHOD Skin samples of 33 regions of the body, as specified in table 1, were taken from the corpse of a Japanese male of 30 years old, who died an accidental death. The samples were fixed in 10 per cent formalin, embedded in celloidin and cut into sections 15 micra thick. The sections were stained with Delafield's hematoxylin and eosin. Observations of sweat glands were made with 2-3 pieces of the skin about 100 sq. -

Modelling Breast Epithelial-Endothelial Interaction in Three-Dimensional Cell Culture
Modelling breast epithelial-endothelial interaction in three-dimensional cell culture A thesis submitted for the degree of Master of Science Sævar Ingþórsson Department of Medicine University of Iceland Instructors and Masters Project Committee: Þórarinn Guðjónsson, Ph.D Magnús Karl Magnússon, MD Kristján Leósson, Ph.D Reykjavik, Iceland September 2008 Samspil æðaþels og eðlilegs og illkynja þekjuvefjar úr brjóstkirtli í þrívíðri frumurækt Ritgerð til meistaragráðu Sævar Ingþórsson Háskóli Íslands Læknadeild Leiðbeinendur og meistaranámsnefnd: Þórarinn Guðjónsson, Ph.D Magnús Karl Magnússon, MD Kristján Leósson, Ph.D Reykjavík, September 2008 Ágrip Brjóstkirtillinn samanstendur af tveimur megingerðum þekjuvefsfruma, kirtilþekju- og vöðvaþekjufrumum. Saman mynda þessar frumugerðir hina greinóttu formgerð brjóstkirtilsins. Kirtilvefurinn er umlukinn æðaríkum stoðvef sem inniheldur margar mismunandi frumugerðir, þ.m.t. bandvefsfrumur og æðaþelsfrumur. Þroskun og sérhæfing kirtilsins er mjög háð samskiptum hans við millifrumuefni brjóstsins og frumur stoðvefjarins. Mest áhersla hefur verið lögð á rannsóknir á bandvefsfrumum í þessu tilliti, en minni athygli beint að æðaþelsfrumum, sem voru lengi taldar gegna því hlutverki einu að miðla súrefni og næringu um líkamann. Á síðustu árum hefur verið sýnt fram á að nýmyndun æða í krabbameinsæxlum spili stórt hlutverk í framþróun æxlisvaxtar og hefur það verið tengt slæmum horfum. Nýlegar rannsóknir hafa sýnt fram á mikilvægt hlutverk æðaþels í þroskun og sérhæfingu ýmissa líffæra, til dæmis í heila, lifur og beinmerg sem og í framþróun krabbameins. Nýleg þekking bendir einnig til mikilvægra áhrifa æðaþels á þroskun eðlilegs og illkynja brjóstvefjar. Markmið verkefnisins er að kanna áhrif brjóstaæðaþels á eðlilegar og illkynja brjóstaþekjufrumulínur og nota til þess þrívíð ræktunarlíkön sem þróuð voru á rannsóknastofunni, sem og að endurbæta þessi líkön til frekari rannsókna á samskiptum æðaþels og þekjufruma. -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -

Periodic Acid-Schiff Positive Material Accumulating Within the Lumen of Eccrine Sweat Glands*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector PERIODIC ACID-SCHIFF POSITIVE MATERIAL ACCUMULATING WITHIN THE LUMEN OF ECCRINE SWEAT GLANDS* GEORGE W. HAMBRICK, JR., M.D. The purpose of this paper is to record findings with regard to diastase-resistant, periodic acid-Schiff positive material in the lumen of eccrine ducts and glands of two individuals. This material has two possible sources in the normal eccrine sweat gland, namely, the cuticle lining of the eccrine duct and the cells of the secretory tubule. Holyoke and Lobits (3) studying 35 normal skin biopsies re- B pp HI D FIG. 1. A. Vertical section through skin of the control area showing dilatation of an eccrine sweat duct with eosinophilic material in the lumen. H. and E., X178. B. Same find- ings as in A above from an area treated daily with 3 per cent hexachloronaphthalene in acetone for one week, Xl7S. C. Section through dermal parts of eccrine duct containing a cast. H. and E., X355. D. Section through dermal eccrine duct containing periodic acid- Schiff positive material in the lumen, X660. *Fromthe Department of Dermatology (Donald M. Pillsbury, M.D., Director), School of Medicine, University of Pennsylvania, Philadelphia 4, Pennsylvania. This study was supported by U. S. Army grant DA-49-007-MD-154. Received for publication March 29, 1957. 213 214 THF JOURNAL OF INVESTIGATIVE DERMATOLOGY ported the presence of material in the lumen of the eccrine duct and gland; they classified the material as amorphous, cast, cellular or bacterial. -

Sweat Gland Myoepithelial Cell Differentiation
Journal of Cell Science 112, 1925-1936 (1999) 1925 Printed in Great Britain © The Company of Biologists Limited 1999 JCS4638 Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture Margarete Schön1,*, Jennifer Benwood1, Therese O’Connell-Willstaedt2 and James G. Rheinwald1,2,‡ 1Division of Dermatology/Department of Medicine, Brigham and Women’s Hospital, and 2Division of Cell Growth and Regulation, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115, USA *Present address: Department of Dermatology, Heinrich-Heine University, Moorenstrasse 5, 40225 Düsseldorf, Germany ‡Author for correspondence (e-mail: [email protected]) Accepted 9 April; published on WWW 26 May 1999 SUMMARY We have characterized precisely the cytokeratin expression myoepithelial cells, a constituent of secretory glands. pattern of sweat gland myoepithelial cells and have Immunostaining of skin sections revealed that only sweat identified conditions for propagating this cell type and gland myoepithelial cells expressed the same pattern of modulating its differentiation in culture. Rare, unstratified keratins and α-sma and lack of E-cadherin as the cell type epithelioid colonies were identified in cultures initiated we had cultured. Interestingly, our immunocytochemical from several specimens of full-thickness human skin. These analysis of ndk, a skin-derived cell line of uncertain cells divided rapidly in medium containing serum, identity, suggests that this line is of myoepithelial origin. epidermal growth factor (EGF), and hydrocortisone, and Earlier immunohistochemical studies by others had found maintained a closely packed, epithelioid morphology when myoepithelial cells to be K7-negative. -
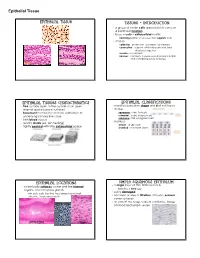
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Sweat Glands • Oil Glands • Mammary Glands
Chapter 4 The Integumentary System Lecture Presentation by Steven Bassett Southeast Community College © 2015 Pearson Education, Inc. Introduction • The integumentary system is composed of: • Skin • Hair • Nails • Sweat glands • Oil glands • Mammary glands © 2015 Pearson Education, Inc. Introduction • The skin is the most visible organ of the body • Clinicians can tell a lot about the overall health of the body by examining the skin • Skin helps protect from the environment • Skin helps to regulate body temperature © 2015 Pearson Education, Inc. Integumentary Structure and Function • Cutaneous Membrane • Epidermis • Dermis • Accessory Structures • Hair follicles • Exocrine glands • Nails © 2015 Pearson Education, Inc. Figure 4.1 Functional Organization of the Integumentary System Integumentary System FUNCTIONS • Physical protection from • Synthesis and storage • Coordination of immune • Sensory information • Excretion environmental hazards of lipid reserves response to pathogens • Synthesis of vitamin D3 • Thermoregulation and cancers in skin Cutaneous Membrane Accessory Structures Epidermis Dermis Hair Follicles Exocrine Glands Nails • Protects dermis from Papillary Layer Reticular Layer • Produce hairs that • Assist in • Protect and trauma, chemicals protect skull thermoregulation support tips • Nourishes and • Restricts spread of • Controls skin permeability, • Produce hairs that • Excrete wastes of fingers and supports pathogens prevents water loss provide delicate • Lubricate toes epidermis penetrating epidermis • Prevents entry of -

Histological Variations in Myoepithelial Cells and Arrectores Pilorum Muscles Among Caudal, Metatarsal and Preorbital Glands In
NOTE Anatomy Histological Variations in Myoepithelial Cells and Arrectores Pilorum Muscles among Caudal, Metatarsal and Preorbital Glands in Hokkaido Sika Deer (Cervus nippon yesoensis Heude, 1884) Nobuo OZAKI1), Masatsugu SUZUKI1)* and Noriyuki OHTAISHI1) 1)Laboratory of Wildlife Biology, The Graduate School of Veterinary Medicine, Hokkaido University, Kita-ku, Sapporo 060–0818, Japan (Received 1 April 2003/Accepted 15 October 2003) ABSTRACT. The morphological characteristics of myoepithelial cells and arrectores pilorum muscles were investigated in caudal, metatarsal and preorbital glands of Hokkaido sika deer (Cervus nippon yesoensis Heude, 1884) using immunohistochemistry for α-smooth muscle actin. In the metatarsal, preorbital and general skin glands, myoepithelial cell layers continuously embraced the secretory epithelium, while in the caudal gland, discontinuous myoepithelial cell rows surrounded the apocrine tubules. There was a trend that the widths of the myoepithelial cells of the caudal and preorbital glands appeared to be thinner than those of the metatarsal and general skin glands. In the metatarsal gland, the arrectores pilorum muscles were highly developed and considerably larger than those in other skin glands. KEY WORDS: arrectores pilorum muscle, myoepithelial cell, sika deer. J. Vet. Med. Sci. 66(3): 283–285, 2004 The specialized skin glands in the cervid species include were incubated for 15 min at room temperature with a bioti- forehead, preorbital, tarsal, metatarsal, interdigital and cau- nylated rabbit antibody against mouse IgG, IgA and IgM dal glands [15], most of which contain both apocrine and (Nichirei). Then they were rinsed three times (10 min each) sebaceous glandular elements [1, 7, 12]. In Hokkaido sika in PBS and incubated for 10 min at room temperature in a deer (Cervus nippon yesoensis Heude, 1884), the existence streptavidin-biotin peroxidase complex (Nichirei).