A Focus on Breast Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients
antibodies Review Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients Andrew T. Lucas 1,2,3,*, Ryan Robinson 3, Allison N. Schorzman 2, Joseph A. Piscitelli 1, Juan F. Razo 1 and William C. Zamboni 1,2,3 1 University of North Carolina (UNC), Eshelman School of Pharmacy, Chapel Hill, NC 27599, USA; [email protected] (J.A.P.); [email protected] (J.F.R.); [email protected] (W.C.Z.) 2 Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] 3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-966-5242; Fax: +1-919-966-5863 Received: 30 November 2018; Accepted: 22 December 2018; Published: 1 January 2019 Abstract: The rapid advancement in the development of therapeutic proteins, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), has created a novel mechanism to selectively deliver highly potent cytotoxic agents in the treatment of cancer. These agents provide numerous benefits compared to traditional small molecule drugs, though their clinical use still requires optimization. The pharmacology of mAbs/ADCs is complex and because ADCs are comprised of multiple components, individual agent characteristics and patient variables can affect their disposition. To further improve the clinical use and rational development of these agents, it is imperative to comprehend the complex mechanisms employed by antibody-based agents in traversing numerous biological barriers and how agent/patient factors affect tumor delivery, toxicities, efficacy, and ultimately, biodistribution. -

Etude Des Résistances Adaptatives Aux Inhibiteurs De Tyrosine Kinase De L’EGFR Dans Les Cancers Bronchiques
Thèse d’exercice Faculté de Pharmacie Année 2020 Thèse N° MÉMOIRE DU DIPLÔME D'ÉTUDES SPÉCIALISÉES D’INNOVATION PHARMACEUTIQUE ET RECHERCHE TENANT LIEU DE THÈSE D’EXERCICE POUR LE DIPLÔME D’ÉTAT DE DOCTEUR ENPHARMACIE Présentée et soutenue publiquement le 24 septembre 2020 par Sarah FIGAROL Née le 19 septembre 1989 à Toulouse Etude des résistances adaptatives aux inhibiteurs de tyrosine kinase de l’EGFR dans les cancers bronchiques Thèse dirigée par Gilles Favre Examinateurs : M. Franck Saint-Marcoux, président du jury M.Gilles Favre, directeur de thèse M. Jean-Marie Canonge, juge M. Julien Mazières, juge M. Antonio Maraver, juge Thèse d’exercice Faculté de Pharmacie Année 2020 Thèse N° MÉMOIRE DU DIPLÔME D'ÉTUDES SPÉCIALISÉES D’INNOVATION PHARMACEUTIQUE ET RECHERCHE TENANT LIEU DE THÈSE D’EXERCICE POUR LE DIPLÔME D’ÉTAT DE DOCTEUR ENPHARMACIE Présentée et soutenue publiquement le 24 septembre 2020 par Sarah FIGAROL Née le 19 septembre 1989 à Toulouse Etude des résistances adaptatives aux inhibiteurs de tyrosine kinase de l’EGFR dans les cancers bronchiques Thèse dirigée par Gilles Favre Examinateurs : M. Franck Saint-Marcoux, président du jury M.Gilles Favre, directeur de thèse M. Jean-Marie Canonge, juge M. Julien Mazières, juge M. Antonio Maraver, juge Sarah FIGAROL | Thèse d’exercice | Université de Limoges |2020 3 Licence CC BY-NC-ND 3.0 Liste des enseignants Le 1er septembre 2019 PROFESSEURS : BATTU Serge CHIMIE ANALYTIQUE CARDOT Philippe CHIMIE ANALYTIQUE ET BROMATOLOGIE DESMOULIERE Alexis PHYSIOLOGIE DUROUX Jean-Luc BIOPHYSIQUE, -

The Role of Biological Therapy in Metastatic Colorectal Cancer After First-Line Treatment: a Meta-Analysis of Randomised Trials
REVIEW British Journal of Cancer (2014) 111, 1122–1131 | doi: 10.1038/bjc.2014.404 Keywords: colorectal; biological; meta-analysis The role of biological therapy in metastatic colorectal cancer after first-line treatment: a meta-analysis of randomised trials E Segelov1, D Chan*,2, J Shapiro3, T J Price4, C S Karapetis5, N C Tebbutt6 and N Pavlakis2 1St Vincent’s Clinical School, University of New South Wales, Sydney, NSW 2052, Australia; 2Royal North Shore Hospital, St Leonards, Sydney, NSW 2065, Australia; 3Monash University and Cabrini Hospital, Melbourne, VIC 3800, Australia; 4The Queen Elizabeth Hospital and University of Adelaide, Woodville South, SA 5011, Australia; 5Flinders University and Flinders Medical Centre, Flinders Centre for Innovation in Cancer, Bedford Park, SA, 5042, Australia and 6Austin Health, VIC 3084, Australia Purpose: Biologic agents have achieved variable results in relapsed metastatic colorectal cancer (mCRC). Systematic meta-analysis was undertaken to determine the efficacy of biological therapy. Methods: Major databases were searched for randomised studies of mCRC after first-line treatment comparing (1) standard treatment plus biologic agent with standard treatment or (2) standard treatment with biologic agent with the same treatment with different biologic agent(s). Data were extracted on study design, participants, interventions and outcomes. Study quality was assessed using the MERGE criteria. Comparable data were pooled for meta-analysis. Results: Twenty eligible studies with 8225 patients were identified. The use of any biologic therapy improved overall survival with hazard ratio (HR) 0.87 (95% confidence interval (CI) 0.82–0.91, Po0.00001), progression-free survival (PFS) with HR 0.71 (95% CI 0.67–0.74, Po0.0001) and overall response rate (ORR) with odds ratio (OR) 2.38 (95% CI 2.03–2.78, Po0.00001). -

IGF System in Sarcomas: a Crucial Pathway with Many Unknowns to Exploit for Therapy
61 1 Journal of Molecular C Mancarella and Targeting IGF system in sarcoma 61:1 T45–T60 Endocrinology K Scotlandi THEMATIC REVIEW 40 YEARS OF IGF1 IGF system in sarcomas: a crucial pathway with many unknowns to exploit for therapy Caterina Mancarella and Katia Scotlandi Experimental Oncology Lab, CRS Development of Biomolecular Therapies, Orthopaedic Rizzoli Institute, Bologna, Italy Correspondence should be addressed to K Scotlandi: [email protected] This paper forms part of a special section on 40 Years of IGF1. The guest editors for this section were Derek LeRoith and Emily Gallagher. Abstract The insulin-like growth factor (IGF) system has gained substantial interest due to its Key Words involvement in regulating cell proliferation, differentiation and survival during anoikis f sarcomas and after conventional and targeted therapies. However, results from clinical trials have f IGF system been largely disappointing, with only a few but notable exceptions, such as trials targeting f targeted therapy sarcomas, especially Ewing sarcoma. This review highlights key studies focusing on IGF f clinical trials signaling in sarcomas, specifically studies underscoring the properties that make this system an attractive therapeutic target and identifies new relationships that may be exploited. This review discusses the potential roles of IGF2 mRNA-binding proteins (IGF2BPs), discoidin domain receptors (DDRs) and metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) in regulating the IGF system. Deeper investigation of these novel regulators of the IGF system may help us to further elucidate the spatial and temporal control of the IGF Journal of Molecular axis, as understanding the control of this axis is essential for future clinical studies. -

Therapeutic Targeting of the IGF Axis
cells Review Therapeutic Targeting of the IGF Axis Eliot Osher and Valentine M. Macaulay * Department of Oncology, University of Oxford, Oxford, OX3 7DQ, UK * Correspondence: [email protected]; Tel.: +44-1865617337 Received: 8 July 2019; Accepted: 9 August 2019; Published: 14 August 2019 Abstract: The insulin like growth factor (IGF) axis plays a fundamental role in normal growth and development, and when deregulated makes an important contribution to disease. Here, we review the functions mediated by ligand-induced IGF axis activation, and discuss the evidence for the involvement of IGF signaling in the pathogenesis of cancer, endocrine disorders including acromegaly, diabetes and thyroid eye disease, skin diseases such as acne and psoriasis, and the frailty that accompanies aging. We discuss the use of IGF axis inhibitors, focusing on the different approaches that have been taken to develop effective and tolerable ways to block this important signaling pathway. We outline the advantages and disadvantages of each approach, and discuss progress in evaluating these agents, including factors that contributed to the failure of many of these novel therapeutics in early phase cancer trials. Finally, we summarize grounds for cautious optimism for ongoing and future studies of IGF blockade in cancer and non-malignant disorders including thyroid eye disease and aging. Keywords: IGF; type 1 IGF receptor; IGF-1R; cancer; acromegaly; ophthalmopathy; IGF inhibitor 1. Introduction Insulin like growth factors (IGFs) are small (~7.5 kDa) ligands that play a critical role in many biological processes including proliferation and protection from apoptosis and normal somatic growth and development [1]. IGFs are members of a ligand family that includes insulin, a dipeptide comprised of A and B chains linked via two disulfide bonds, with a third disulfide linkage within the A chain. -

Correlation Between Gene Expression of IGF-1R Pathway Markers and Cetuximab Benefit in Metastatic Colorectal Cancer
Published OnlineFirst January 31, 2012; DOI: 10.1158/1078-0432.CCR-11-1135 Clinical Cancer Predictive Biomarkers and Personalized Medicine Research Correlation between Gene Expression of IGF-1R Pathway Markers and Cetuximab Benefit in Metastatic Colorectal Cancer Fei Huang, Li-an Xu, and Shirin Khambata-Ford Abstract Purpose: This study examined potential correlations between markers related to the insulin-like growth factor-1 receptor (IGF-1R) pathway and clinical benefit from the anti–epidermal growth factor receptor (EGFR) monoclonal antibody cetuximab in metastatic colorectal cancer (mCRC). Experimental Design: Gene expression profiles for 70 pretreatment specimens from metastatic lesions of patients with chemorefractory mCRC receiving cetuximab monotherapy were analyzed using 74 predefined Gene-Chip probesets representing 33 unique IGF-1R pathway markers to determine correlations with progression-free survival (PFS) and disease control rate. Results: Higher IGF-1R, higher GRB7, and lower INSIG2 expression were associated with longer PFS with cetuximab in univariate analyses, particularly in patients with wild-type K-Ras tumors: median, 122 versus 60 days (P ¼ 0.01), 122 versus 57 days (P ¼ 0.011), and 57 versus 156 days (P < 0.0001), favoring higher IGF- 1R, higher GRB7, and lower INSIG2 expression, respectively. Lower IGF-1 expression was associated with a PFS benefit with cetuximab, whereas lower IGFBP3 and INSR expression levels showed trends for a PFS benefit. Lower INSIG2 expression (vs. higher expression) was associated with greater PFS in the high epiregulin-expressing group (P ¼ 0.001), but not in the low-expressing cohort suggesting an effect independent from the previously reported effect of epiregulin expression. -

Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy
cancers Review Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy Laura Boyero 1 , Amparo Sánchez-Gastaldo 2, Miriam Alonso 2, 1 1,2,3, , 1,2, , José Francisco Noguera-Uclés , Sonia Molina-Pinelo * y and Reyes Bernabé-Caro * y 1 Institute of Biomedicine of Seville (IBiS) (HUVR, CSIC, Universidad de Sevilla), 41013 Seville, Spain; [email protected] (L.B.); [email protected] (J.F.N.-U.) 2 Medical Oncology Department, Hospital Universitario Virgen del Rocio, 41013 Seville, Spain; [email protected] (A.S.-G.); [email protected] (M.A.) 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), 28029 Madrid, Spain * Correspondence: [email protected] (S.M.-P.); [email protected] (R.B.-C.) These authors contributed equally to this work. y Received: 16 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. This has led to the development of several therapeutic strategies focused in this direction. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease. Abstract: After several decades without maintained responses or long-term survival of patients with lung cancer, novel therapies have emerged as a hopeful milestone in this research field. -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -
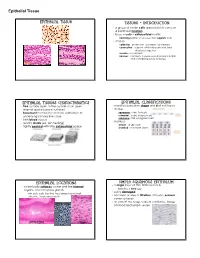
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Progress in Pancreatic Ductal Adenocarcinoma (PDAC) Research Working Group (PDAC Progress Working Group)
National Cancer Institute Clinical Trials and Translational Research Advisory Committee (CTAC) Progress in Pancreatic Ductal Adenocarcinoma (PDAC) Research Working Group (PDAC Progress Working Group) Working Group Report March 6, 2019 The report was accepted at the March 6, 2019 CTAC Meeting ______________________________________________________________________________________________________ PDAC Progress WG Report: Accepted by the Clinical Trials and Translational Research Advisory Committee on March 6, 2019 Page 1 TABLE OF CONTENTS Page National Cancer Institute Clinical Trials and Translational Research Advisory Committee (CTAC) Progress in Pancreatic Ductal Adenocarcinoma (PDAC) Research 3 Working Group (PDAC Progress Working Group) Report Appendix 1: Progress in Pancreatic Ductal Adenocarcinoma (PDAC) Research 16 Working Group (PDAC Progress WG) 2018 Roster Appendix 2: Funded Project Summary FY 2015 – FY 2017 • NIH PDAC Funded Projects, Initiative 1: Understanding the 18 Biological Relationship Between PDAC and Diabetes Mellitus • NIH PDAC Funded Projects, Initiative 2: Evaluating Longitudinal Screening Protocols, for Biomarkers for Earl Detection of PDAC 20 and its Precursors • NIH PDAC Funded Projects, Initiative 3: Study New Therapeutic 28 Strategies in Immunotherapy • NIH PDAC Funded Projects, Initiative 4: Developing New Treatment Approaches that Interfere with RAS Oncogene- 33 Dependent Signaling Pathways • NIH PDAC Funded Projects, No Related to the Framework t 38 Initiatives Appendix 3: PDAC Publications Supported by Grants -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell.