A New Look at Transudation: the Apocrine Connection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Efferosome Maturation and the Processing of Apoptotic Bodies
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-15-2014 12:00 AM Characterization of Efferosome Maturation and the Processing of Apoptotic Bodies Yohan Kim The University of Western Ontario Supervisor Dr. Bryan Heit The University of Western Ontario Graduate Program in Microbiology and Immunology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Yohan Kim 2014 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Cell Biology Commons, Immunity Commons, and the Other Immunology and Infectious Disease Commons Recommended Citation Kim, Yohan, "Characterization of Efferosome Maturation and the Processing of Apoptotic Bodies" (2014). Electronic Thesis and Dissertation Repository. 2268. https://ir.lib.uwo.ca/etd/2268 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. CHARACTERIZATION OF EFFEROSOME MATURATION AND THE PROCESSING OF APOPTOTIC BODIES (Thesis format: Monologue) by Yohan Kim Graduate Program in Microbiology and Immunology A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada © Yohan Kim 2014 Abstract Every day billions of cells in our bodies undergo apoptosis and are cleared through efferocytosis – a phagocytosis-like process in which phagocytes engulf and degrade apoptotic cells. Proper processing of efferosomes prevents inflammation and immunogenic presentation of antigens. -

Transcytosis of Ngcam in Epithelial Cells Reflects Differential Signal
Published August 8, 2005 JCB: ARTICLE Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways Eric Anderson, Sandra Maday, Jeff Sfakianos, Michael Hull, Bettina Winckler, David Sheff, Heike Fölsch, and Ira Mellman Department of Cell Biology, Ludwig Institute for Cancer Research, Yale University School of Medicine, New Haven, CT 06520 gCAM is a cell adhesion molecule that is largely ability to interact with clathrin adaptors. Based on the be- axonal in neurons and apical in epithelia. In Ma- havior of various NgCAM mutants, it appears that after Ndin-Darby canine kidney cells, NgCAM is tar- arrival at the basolateral surface, the AP-1B interaction geted to the apical surface by transcytosis, being first in- site is silenced by phosphorylation of Tyr33. This slows en- serted into the basolateral domain from which it is docytosis and inhibits basolateral recycling from endo- internalized and transported to the apical domain. Initial somes, resulting in NgCAM transcytosis due to a cryptic basolateral transport is mediated by a sequence motif apical targeting signal in its extracellular domain. Thus, Downloaded from (Y33RSL) decoded by the AP-1B clathrin adaptor complex. transcytosis of NgCAM and perhaps other membrane This motif is a substrate in vitro for tyrosine phosphoryla- proteins may reflect the spatial regulation of recognition tion by p60src, a modification that disrupts NgCAM’s by adaptors such as AP-1B. on March 10, 2017 Introduction The generation and maintenance of cellular polarity is an es- ously resorted in endosomes to maintain their polarized distri- sential aspect of multicellular life. -

Apical-To-Basolateral Transcytosis of Photoreceptor Outer Segments Induced by Lipid Peroxidation Products in Human Retinal Pigment Epithelial Cells
Retinal Cell Biology Apical-to-Basolateral Transcytosis of Photoreceptor Outer Segments Induced by Lipid Peroxidation Products in Human Retinal Pigment Epithelial Cells Tim U. Krohne,1 Frank G. Holz,1 and Ju¨rgen Kopitz2 PURPOSE. Progressive accumulation of extracellular material at posit formation and drusen biogenesis in AMD. (Invest Oph- the basolateral side of the retinal pigment epithelium (RPE) is thalmol Vis Sci. 2010;51:553–560) DOI:10.1167/iovs.09-3755 a key event in the pathogenesis of age-related macular degen- eration (AMD). The authors previously demonstrated that mod- rogressive deposition of extracellular material between the ifications with lipid peroxidation products, such as 4-hy- basolateral side of the retinal pigment epithelium (RPE) droxynonenal (HNE) and malondialdehyde (MDA), stabilize P and adjacent Bruch’s membrane is a hallmark of early-stage photoreceptor outer segment (POS) proteins against lysosomal age-related macular degeneration (AMD).1,2 Among the various degradation. Herein, they tested RPE cells for the basolateral histologically and clinically distinguishable manifestations of release of undegraded modified POS proteins. sub-RPE deposits, basal linear deposits (BLinD) and soft drusen METHODS. Polarized cultures of the human RPE cell line are recognized as specific for AMD.3,4 Both BLinD and soft ARPE-19 on permeable membranes were incubated with io- drusen represent accumulations of material described as mem- dine-125–labeled POS on the apical side. After 24 hours, radio- branous debris5 between the RPE basement membrane and the activity was quantified in apical medium, cell lysates, and inner collagenous layer of Bruch’s membrane and are, there- basolateral medium after separation of undegraded proteins by fore, considered diffuse and focal manifestations, respectively, precipitation. -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -

Thyroid Hormone Synthesis Continues Despite Biallelic Thyroglobulin Mutation with Cell Death
Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death Xiaohan Zhang, … , Viviana A. Balbi, Peter Arvan JCI Insight. 2021. https://doi.org/10.1172/jci.insight.148496. Research In-Press Preview Endocrinology Graphical abstract Find the latest version: https://jci.me/148496/pdf Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death Authors: Xiaohan Zhang1, Aaron P. Kellogg1, Cintia E. Citterio1,2,3, Hao Zhang1, Dennis Larkin1, Yoshiaki Morishita1,4, Héctor M. Targovnik2,3, Viviana Balbi5, and Peter Arvan1* Affiliations: 1Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48105 2Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Departamento de Microbiología, Inmunología, Biotecnología y Genética/Cátedra de Genética. Buenos Aires, Argentina. 3CONICET, Universidad de Buenos Aires, Instituto de Inmunología, Genética y Metabolismo (INIGEM), Buenos Aires, Argentina 4Division of Diabetes, Department of Internal Medicine, Aichi Medical University, 1-1 Yazakokarimata, Nagakute, Aichi 480-1195, Japan 5Department of Endocrinology and Growth, Hospital de Niños Sor María Ludovica, La Plata, Argentina Conflict of interest: The authors declare that no conflict of interest exists. Short Title: Thyroxine synthesis from dead cells 1 Complete absence of thyroid hormone is incompatible with life in vertebrates. Thyroxine is synthesized within thyroid follicles upon iodination of thyroglobulin conveyed from the endoplasmic reticulum (ER), via the Golgi complex, to the extracellular follicular lumen. In congenital hypothyroidism from bi-allelic thyroglobulin mutation, thyroglobulin is misfolded and cannot advance from the ER, eliminating its secretion and triggering ER stress. Nevertheless, untreated patients somehow continue to synthesize sufficient thyroxine to yield measurable serum levels that sustain life. -
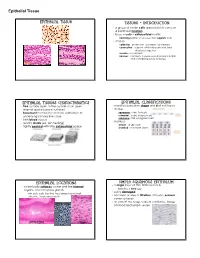
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

A Focus on Breast Cancer
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Pharmacol Manuscript Author Ther. Author Manuscript Author manuscript; available in PMC 2017 May 01. Published in final edited form as: Pharmacol Ther. 2016 May ; 161: 79–96. doi:10.1016/j.pharmthera.2016.03.003. Emerging therapeutic targets in metastatic progression: a focus on breast cancer Zhuo Li and Yibin Kang* Department of Molecular Biology, Princeton University, Princeton, NJ, 08544, United States Abstract Metastasis is the underlying cause of death for the majority of breast cancer patients. Despite significant advances in recent years in basic research and clinical development, therapies that specifically target metastatic breast cancer remain inadequate, and represents the single greatest obstacle to reducing mortality of late-stage breast cancer. Recent efforts have leveraged genomic analysis of breast cancer and molecular dissection of tumor-stromal cross-talk to uncover a number of promising candidates for targeted treatment of metastatic breast cancer. Rational combinations of therapeutic agents targeting tumor-intrinsic properties and microenvironmental components provide a promising strategy to develop precision treatments with higher specificity and less toxicity. In this review, we discuss the emerging therapeutic targets in breast cancer metastasis, from tumor-intrinsic pathways to those that involve the host tissue components, including the immune system. Keywords Breast cancer; Metastasis; Targeted therapy; Tumor microenvironment; Immunotherapy 1. Introduction The overall 5-year survival rate for breast cancer currently stands at 90% — a dramatic improvement over the 63% survival rate in the early 1960s. When stratified by stage, the 5- year survival rates have increased to 99% for localized disease and 85% for regional advanced disease, a trend that can be attributed to early diagnoses and better treatment regimens. -

Transcytosis of Trka Leads to Diversification of Dendritic Signaling
www.nature.com/scientificreports OPEN Transcytosis of TrkA leads to diversifcation of dendritic signaling endosomes Received: 8 January 2018 Kelly Barford 1, Austin Keeler2, Lloyd McMahon1, Kathryn McDaniel1, Chan Choo Yap1, Accepted: 5 March 2018 Christopher D. Deppmann2,3 & Bettina Winckler1 Published: xx xx xxxx The development of the peripheral nervous system relies on long-distance signaling from target organs back to the soma. In sympathetic neurons, this long-distance signaling is mediated by target derived Nerve Growth Factor (NGF) interacting with its axonal receptor, TrkA. This ligand receptor complex internalizes into what is commonly referred to as the signaling endosome which is transported retrogradely to the soma and dendrites to mediate survival signaling and synapse formation, respectively. The molecular identity of signaling endosomes in dendrites has not yet been determined. Here, we perform a detailed analysis of TrkA endosomal compartments and trafcking patterns. We fnd that signaling endosomes are not uniform but molecularly diversifed into Rab7 (late endosome) and Rab11 (recycling endosome) populations in axons and dendrites in vitro and in the soma in vivo. Surprisingly, TrkA-NGF signaling endosomes in dendrites undergo dynamic trafcking events, including putative fusion and fssion. Overall, we fnd that signaling endosomes do not remain as a singular endosomal subtype but instead exist in multiple populations that undergo dynamic endosomal trafcking events. These dynamic events might drive functional diversifcation of the signaling endosome. Te development of the sympathetic and sensory nervous systems requires the target derived neurotrophic factor, Nerve Growth Factor (NGF)1. Te molecular and cellular mechanisms that enable target derived NGF to transmit signals long distances from distal axons to the cell body have been under intense investigation for several years. -

Volume 21 • 2015
Weill Co rnell Medical College Volume 21 • January 2015 The Newsletter of the Department of Pathology and Laboratory Medicine at NewYork-Presbyterian Hospital/Weill Cornell Medical Center Contents Research Highlights Localization of Annexin A5 on the surface of placental trophoblasts 1 by David P. Hajjar, PhD Research Highlights 2 Focus (green: AnxA5; blue: nucleus) Figure 1 : Expression of AnxA5 (green fluorescence) on 3 cultured syncytialized human placental trophoblasts. Clinical Pathology Update Blue (DAPI) fluorescence marks the nuclei. [bars = 50 m. Confocal microscopy 3-dimensional projections of Z-axis image stacks, voxel size ( m): width 0.73, height 0.73, and 4-6 Drs. Jacob Rand and Xiao-Xuan Wu. depth 1.50] (for complete description, see Wu XX, Guller S, The Department is delighted to welcome Dr. Jacob Rand , Rand JH. Hydroxychloroquine reduces binding of antiphos - Keynotes pholipid antibodies to syncytiotrophoblasts and restores Professor of Pathology and Laboratory Medicine, as Vice annexin A5 expression. Am J Obstet Gynecol . 2011 Dec; Chairman for Laboratory Medicine and Director of the Clinical 205(6):576.e7-14.) 7 Laboratories. Pathology Faculty’s Dr. Rand comes to us from Montefiore Medical Center and Global Travels the Albert Einstein College of Medicine where he served as Atomic force Microscopy: Structure of the Director of Hematology, Advanced Coagulation and Protein aPL Ig- 2GPI Complexes Separation Laboratories and as Professor of Pathology, 8 Medicine, and Obstetrics and Gynecology, and Director of the -HCQ Resident’s Corner Hematology Laboratories. Dr. Rand’s record of achievement spans across disciplines, with strong backgrounds as a clini cal physician, clinical laboratory director, teacher and 9-11 +HCQ basic scientist. -

Epithelial Cells Inhibit HIV-1 Transcytosis Across Human HIV-1
Mucosal and Plasma IgA from HIV-1-Exposed Uninfected Individuals Inhibit HIV-1 Transcytosis Across Human Epithelial Cells This information is current as of September 30, 2021. Claudia Devito, Kristina Broliden, Rupert Kaul, Lennart Svensson, Kari Johansen, Peter Kiama, Joshua Kimani, Lucia Lopalco, Stefania Piconi, Job J. Bwayo, Francis Plummer, Mario Clerici and Jorma Hinkula J Immunol 2000; 165:5170-5176; ; Downloaded from doi: 10.4049/jimmunol.165.9.5170 http://www.jimmunol.org/content/165/9/5170 http://www.jimmunol.org/ References This article cites 39 articles, 10 of which you can access for free at: http://www.jimmunol.org/content/165/9/5170.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 30, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Mucosal and Plasma IgA from HIV-1-Exposed Uninfected Individuals Inhibit HIV-1 Transcytosis Across Human Epithelial Cells1 Claudia Devito,* Kristina Broliden,2* Rupert Kaul,† Lennart Svensson,‡ Kari Johansen,‡ Peter Kiama,† Joshua Kimani,† Lucia Lopalco,§ Stefania Piconi,¶ Job J. -

Endocytic Deficiency Induced by ITSN-1S Knockdown Alters the Smad2/3-Erk1/2 Signaling Balance Downstream of Alk5
ß 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 1528–1541 doi:10.1242/jcs.163030 RESEARCH ARTICLE Endocytic deficiency induced by ITSN-1s knockdown alters the Smad2/3-Erk1/2 signaling balance downstream of Alk5 Cristina Bardita1, Dan N. Predescu1,2, Fei Sha1, Monal Patel1, Ganesh Balaji3 and Sanda A. Predescu1,2,* ABSTRACT leading to pulmonary edema, evidence suggests that apoptosis plays a beneficial role during ALI resolution owing to the pro- Recently, we demonstrated in cultured endothelial cells and in vivo regenerative role of clearance of apoptotic cells (Predescu et al., that deficiency of an isoform of intersectin-1, ITSN-1s, impairs 2013; Schmidt and Tuder, 2010). This effect is mediated through caveolae and clathrin-mediated endocytosis and functionally the production of growth factors including TGFb by macrophages upregulates compensatory pathways and their morphological engulfing apoptotic cells, or perhaps by other vascular cells carriers (i.e. enlarged endocytic structures, membranous rings or (Bardita et al., 2013; Henson and Tuder, 2008). TGFb, owing to tubules) that are normally underrepresented. We now show that its anti-inflammatory properties confines the extent of septal these endocytic structures internalize the broadly expressed injury and speeds recovery in ALI (D’Alessio et al., 2009). transforming growth factor b receptor I (TGFb-RI or TGFBR1), We have recently shown that in vivo deficiency of ITSN-1s, an also known as Alk5, leading to its ubiquitylation and degradation. isoform of ITSN-1 that is highly prevalent in lung endothelium and Moreover, the apoptotic or activated vascular cells of the ITSN-1s- deficiency of which is relevant to the pathology of ALI/ARDS knockdown mice release Alk5-bearing microparticles to the (Bardita et al., 2013; Predescu et al., 2013), induces extensive lung systemic circulation. -

(Eccrine and Apocrine Glands) During Childhood in Japanese, Especially on the PAS Positive Substance and Iron
Histochemical Investigation on the Axillary Sweat Glands (Eccrine and Apocrine Glands) during Childhood in Japanese, especially on the PAS Positive Substance and Iron By Iwao Yasui and Hiroshi Kagemoto Department of Anatomy, School of Medicine, Keio University Shinjuku, Tokyo, Japan (Director : Prof. Dr. T. Taniguchi) Introduction The application of periodic acid to histochemical study on poly- saccharide was made for the first time by Hot c h k i s s, M c Man u s, Lillie et G r e co and Marches e. This histochemical method was called for short PAS method " (namely, Periodic Acid S c h i f f). 'Recently , a good deal of the histochemical studies regarding glyco- gen, mucopolysaccharide and other substances in the sweat glands have been made by such various scholars as Bunt in g, Wislocki and Dempsey (1948), Montagna, Chase and Lobitz (1952), Montagna, Chase and M e l a r a g n o (1951) and others, apply- ing the PAS method. On the other hand, studies on the iron reaction in the sweat glands have been numerously reported since H o m m a (1925)'s research on the axillary sweat glands by Turnbull reaction, that is, Klaar (1926), Herzenberg (1927), Moriyama (1927), Oono and Kinoshita (1927), Wosllard (1930), Richter (1933), Manca (1934), Way and Memmesheimer (1938), Yoshi- mura (1942), Nagamitsu (1941), Yoshihiro (1942a), Cavaz- zana (1947), Bunting, Wislocki and Dempsey (1948),Iwa- shige (1951),Montagna,Chase and Lobitz (1953) and others. Nevertheless, there have been no reports which give histochemical observation on the axillary sweat glands during childhood by age group.