1 EDF S1 Guidelines on the Management of Lichen Planus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atypical Variants of Mycosis Fungoides
Altınışık et al. Atypical Variants of Mycosis Fungoides REVIEW DOI: 10.4274/jtad.galenos.2021.09719 J Turk Acad Dermatol 2021;15(2):30-33 Atypical Variants of Mycosis Fungoides Dursun Dorukhan Altınışık, Özge Aşkın, Tuğba Kevser Uzunçakmak, Burhan Engin Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Dermatology, Istanbul, Turkey ABSTRACT Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL) and it is characterised by specific histological features. MF is in a spectrum of disorders which includes Sezary syndrome and other CTCL subtypes. Clinically and histopathologically it can imitate other T-cell mediated dermatosis hence making its diagnosis difficult. Due to different prognosis and difference in treatment strategies other subtypes of CTCL must be differentiated from MF. MF is a clinically indolent low-grade lymphoma which is seen in people aged between 55- 60 and it’s seen more commonly in men (2:1 ratio). Meanwhile other subtypes of MF like folliculotropic variant may show relatively a more aggressive course and our treatment strategy may differ. Keywords: Mycosis fungoides, Cutaneous T-cell lymphoma, Phototherapy, PUVA Introduction follicles frequently causing alopecia and in the paediatric population Mycosis fungoides (MF) is the most common type of cutaneous T-cell it tends to accompany hypopigmented patches and plaques. FMF lymphoma (CTCL) and it is characterised by specific histological preferentially involves the head and neck region however it can features [1]. MF is in a spectrum of disorders which includes Sezary sometimes involve the trunk or the extremities [6]. Patients with syndrome and other CTCL subtypes. Clinically and histopathologically FMF have significant pruritus which may be overlapped by S. -

Lichen Spinulosus: Case Report and Review of Literatures
Journal of Health Science 2015, 5(3A): 20-22 DOI: 10.5923/s.health.201501.07 Lichen Spinulosus: Case Report and Review of Literatures Khalid Al Hawsawi1,*, Kholood Almehmadi2, Bushra Alraddadi3, Ohood Aljuhani2 1Dermatology Consultant, Head of Dermatology Department, King Abdul Aziz Hospital, Makkah, Saudi Arabia 2Medical intern, King Abdul Aziz Hospital, Makkah, Saudi Arabia 3Dermatology Resident, King Abdul Aziz Hospital, Makkah, Saudi Arabia Abstract Lichen Spinulosus is a rare childhood disease. It is characterized by follicular hyperkeratotic papules that may coalesce into plaques. Individual lesion shows conical projection, hair like horny spine. The etiology is unknown but genetic predisposition has been suggested as a possible cause. It has a tendency to disappear spontaneously at puberty. Herein we present a 12-year-old boy presented with 6- month- history of persistent asymptomatic skin lesions. Skin examinations revealed multiple grouped scaly, hypopigmented tiny follicular papules with horny spines on his back. Soles showed planter keratoderma. Skin biopsy showed dilated hair follicle filled with keratin plugging. Diagnosis of LS associated with planter keratoderma was made. The patient responded well to topical lactic acid (12%), urea (20-30%), and betamethasone valerate (0.1%) preparations. Keywords Lichen spinulosa, Lichen Spinulosus 1. Introduction appearance. LS usually involves extensor surfaces of arms, trochanteric regions, neck, abdomen, buttocks, thigh, Lichen spinulosus (LS) is a genetic disorder of popliteal fossae, and knees. The histopathology of the lesion is not specific showing dilated hair follicle with keratin keratinization of hair follicles. LS was first described by Adamson in 1908. [1] It is a member of the family of plugging. -

Differentiating Keratosis Follicularis Spinulosa Decalvans from Monilithrix : Two Rare Causes of Scarring Alopecia in Young Male Siblings
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 18, Issue 5 Ser. 7 (May. 2019), PP 01-03 www.iosrjournals.org Differentiating Keratosis Follicularis Spinulosa Decalvans From Monilithrix : Two Rare Causes Of Scarring Alopecia In Young Male Siblings. 1 2 3 4 5 Heena Singdia , Rohit Garg , Sinni Jain , Vijay Paliwal , Puneet Bhargava , Deepak Mathur6 1,2,3,4,5,6(Department of dermatology, venereology and leprology, SMS Medical College, India) Corresponding Author: Heena Singdia Abstract: Keratosis follicularis spinulosa decalvans (KFSD) is a rare disorder affecting the hair follicles characterized by progressive cicatricial alopecia of the scalp and eyebrows, with usually X-linked recessive mode of transmission hence predominantly affecting Males.[1] Monilethrix is a rare structural Hair shaft disorder with autosomal dominant mode of transmission, characterized by short, fragile, brittle hair that breaks spontaneously resulting in patchy dystrophic alopecia.[2] Key words: Keratosis Follicularis Spinulosa Decalvans, Monilithrix, Hereditary Scarring Alopecias --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 02-05-2019 Date of acceptance: 16-05-2019 --------------------------------------------------------------------------------------------------------------------------------------------------- I. Introduction KFSD is a rare type of primary scarring alopecia with lymphocytic predominance. -
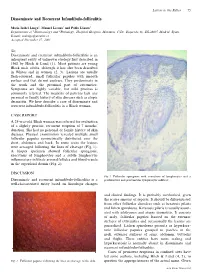
Disseminate and Recurrent Infundibulo-Folliculitis
Letters to the Editor 75 Disseminate and Recurrent Infundibulo-folliculitis Mar´õ a Isabel Longo1, Manuel Lecona2 and Pablo La´zaro1 Departments of 1Dermatology and 2Pathology, Hospital Grogorio Maran˜on, C/Dr. Esquerdo 46, ES-28007 Madrid, Spain. E-mail: [email protected] Accepted December 17, 2001. Sir, Disseminate and recurrent infundibulo-folliculitis is an infrequent entity of unknown etiology rst described in 1968 by Hitch & Lund (1). Most patients are young Black male adults, although it has also been described in Whites and in women (2, 3). Lesions are usually esh-coloured, small follicular papules with smooth surface and that do not coalesce. They predominate in the trunk and the proximal part of extremities. Symptoms are highly variable, but mild pruritus is commonly referred. The majority of patients lack any personal or family history of skin diseases such as atopic dermatitis. We here describe a case of disseminate and recurrent infundibulo-folliculitis in a Black woman. CASE REPORT A 25-year-old Black woman was referred for evaluation of a slightly pruritic, recurrent eruption of 7 months’ duration. She had no personal or family history of skin diseases. Physical examination revealed multiple small follicular papules symmetrically distributed over the chest, abdomen and back. In some areas the lesions were arranged following the lines of cleavage (Fig. 1). A biopsy specimen showed follicular spongiosis, exocytosis of lymphocytes and a subtle lymphocytic in ammatory in ltrate around follicles and blood vessels in the super cial dermis (Fig. 2). DISCUSSION Fig. 2. Follicular spongiosis with exocytosis of lymphocytes and a Disseminate and recurrent infundibulo-folliculitis is a perifollicular and perivascular lymphocytic in ltrate. -

Aafp Fmx 2020
Challenging Pediatric Rashes Richard P. Usatine, MD, FAAFP Professor, Family and Community Medicine Professor, Dermatology and Cutaneous Surgery University of Texas Health, San Antonio 1 ACTIVITY DISCLAIMER The material presented here is being made available by the American Academy of Family Physicians for educational purposes only. Please note that medical information is constantly changing; the information contained in this activity was accurate at the time of publication. This material is not intended to represent the only, nor necessarily best, methods or procedures appropriate for the medical situations discussed. Rather, it is intended to present an approach, view, statement, or opinion of the faculty, which may be helpful to others who face similar situations. The AAFP disclaims any and all liability for injury or other damages resulting to any individual using this material and for all claims that might arise out of the use of the techniques demonstrated therein by such individuals, whether these claims shall be asserted by a physician or any other person. Physicians may care to check specific details such as drug doses and contraindications, etc., in standard sources prior to clinical application. This material 2 might contain recommendations/guidelines developed by other organizations. Please note that although these guidelines might be included, this does not necessarily imply the endorsement by the AAFP. 2 1 Disclosure Statement It is the policy of the AAFP that all individuals in a position to control content disclose any relationships with commercial interests upon nomination/invitation of participation. Disclosure documents are reviewed for potential conflicts of interest. If conflicts are identified, they are resolved prior to confirmation of participation. -
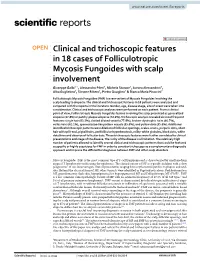
Clinical and Trichoscopic Features in 18 Cases of Folliculotropic Mycosis Fungoides with Scalp Involvement
www.nature.com/scientificreports OPEN Clinical and trichoscopic features in 18 cases of Folliculotropic Mycosis Fungoides with scalp involvement Giuseppe Gallo1*, Alessandro Pileri2, Michela Starace2, Aurora Alessandrini2, Alba Guglielmo2, Simone Ribero1, Pietro Quaglino1 & Bianca Maria Piraccini2 Folliculotropic Mycosis Fungoides (FMF) is a rare variant of Mycosis Fungoides involving the scalp leading to alopecia. The clinical and trichoscopic features in 18 patients were analyzed and compared with the reports in the literature. Gender, age, disease stage, site of onset were taken into consideration. Clinical and trichoscopic analyses were performed on each patient. From a clinical point of view, Folliculotropic Mycosis Fungoides lesions involving the scalp presented as generalized alopecia (27.8%) or patchy-plaque alopecia (72.2%). Trichoscopic analysis revealed six most frequent features: single hair (83.3%), dotted dilated vessels (77.8%), broken-dystrophic hairs (66.7%), vellus hairs (61.1%), spermatozoa-like pattern vessels (55.6%), and yellow dots (55.6%). Additional identifed trichoscopic patterns were dilation of follicular openings, scales-crusts, purpuric dots, short hair with split-end, pigtail hairs, perifollicular hyperkeratosis, milky-white globules, black dots, white dots/lines and absence of follicular dots. These trichoscopic features were further correlated to clinical presentations and stage of the disease. The rarity of the disease is a limitation. The relatively high number of patients allowed to identify several clinical and trichoscopic patterns that could be featured as specifc or highly suspicious for FMF in order to consider trichoscopy as a complementary diagnostic approach and improve the diferential diagnoses between FMF and other scalp disorders. Mycosis Fungoides (MF) is the most common type of T-cell lymphoma and is characterized by small-medium atypical T lymphocytes infltrating the epidermis. -
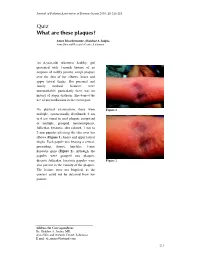
Quiz What Are These Piaques?
Journal of Pakistan Association of Dermatologists 2003; 13 : 211-213. Quiz What are these pIaques? Amor Khachemoune, Shahbaz A Janjua Ayza Skin and Research Centre , Lalamusa An 8-year-old, otherwise healthy, girl presented with 1-month history of an eruption of mildly pruritic, rough plaques over the skin of her elbows, knees and upper lateral thighs. Her personal and family medical histories were unremarkable; particularly there was no history of atopic diathesis. She denied the use of any medication in the recent past. On physical examination, there were Figure 1 multiple, symmetrically distributed, 2 cm to 6 cm round to oval plaques comprised of multiple, grouped, monomorphous, follicular, keratotic, skin colored, 1 mm to 2 mm papules affecting the skin over her elbows ( Figure 1 ), knees and upper lateral thighs. Each papule was bearing a central, protruding, thorny, hair-like, 1-mm keratotic spine ( Figure 2 ). Although, the papules were grouped into plaques, discrete follicular, keratotic papules were Figure 2 also present in the vicinity of the plaques. The lesions were not biopsied, as the consent could not be obtained from her parents. Address for Correspondence Dr. Shahbaz A. Janjua, MD, Ayza Skin and research Centre, Lalamusa. E mail: [email protected] 211 What are these plaques? Amor Khachemoune & S.A Janjua . Diagnosis On histopathology, LS lesions show a Lichen spinulosus central, infundibular, keratotic plug dilating the follicle and protruding above Lichen spinulosus (LS) is a rare, the epidermal surface. Follicular idiopathic, follicular, keratotic dermatosis hyperkeratosis, parakeratosis and occurring mostly in children, adolescents acanthosis may be present. Dermal and young adults. -

“Why Do I Have Goose-Like Flesh?”
PHOTO CLINIC A Brief Photo-Based Case “Why do I have goose-like flesh?” Catherine Lagacé, MD A 24-year-old female seeks medical advice for the poor cosmetic appearance of her skin. She is concerned about the rough texture and goose- flesh look of her outer arms and anterior thighs which, according to her, have been present since before puberty. Her past medical history is negative. She has tried many moisturizers over the years, which have failed to improve her condition substan- tially. She wonders if a special cream is avail- able for this condition. What do you diagnose? This is a case of keratosis pilaris. It is a very common benign disorder, arising from the exces- sive accumulation of keratin at the follicular ostium. It affects approximately 50% to 80% of ado- lescents and 40% of adults, half of which have a positive family history of keratosis pilaris. An autosomal dominant inheritance with variable penetrance has been described. Every racial group is equally susceptible but Figure 1. Keratosis pilaris. females may be more frequently affected than males. crete papule. Lesions are usually asymptomatic, Keratosis pilaris is often described in associa- although some may complain of occasional pru- tion with ichthyosis vulgaris and atopic dermatitis. ritus. Inflammation may or may not be present. Keratosis pilaris manifests as small folliculo- Some improvement can be seen during the centric keratotic papules that most commonly summer months, while worsening in winter is involve the posterolateral aspect of the upper not infrequent. Keratosis pilaris tends to get bet- arms, the anterior thighs and the cheeks. -

Acne and Related Conditions
Rosanne Paul, DO Madeline Tarrillion, DO Miesha Merati, DO Gregory Delost, DO Emily Shelley, DO Jenifer R. Lloyd, DO, FAAD American Osteopathic College of Dermatology Disclosures • We do not have any relevant disclosures. Cleveland before June 2016 Cleveland after June 2016 Overview • Acne Vulgaris • Folliculitis & other – Pathogenesis follicular disorders – Clinical Features • Variants – Treatments • Rosacea – Pathogenesis – Classification & clinical features • Rosacea-like disorders – Treatment Acne vulgaris • Pathogenesis • Multifactorial • Genetics – role remains uncertain • Sebum – hormonal stimulation • Comedo • Inflammatory response • Propionibacterium acnes • Hormonal influences • Diet Bolognia et al. Dermatology. 2012. Acne vulgaris • Clinical Features • Face & upper trunk • Non-inflammatory lesions • Open & closed comedones • Inflammatory lesions • Pustules, nodules & cysts • Post-inflammatory hyperpigmentation • Scarring • Pitted or hypertrophic Bolognia et al. Dermatology. 2012. Bolognia et al. Dermatology. 2012. Acne variants • Acne fulminans • Acne conglobata • PAPA syndrome • Solid facial edema • Acne mechanica • Acne excoriée • Drug-induced Bolognia et al. Dermatology. 2012. Bolognia et al. Dermatology. 2012. Bolognia et al. Dermatology. 2012. Bolognia et al. Dermatology. 2012. Acne variants • Occupational • Chloracne • Neonatal acne (neonatal cephalic pustulosis) • Infantile acne • Endocrinological abnormalities • Apert syndrome Bolognia et al. Dermatology. 2012. Bolognia et al. Dermatology. 2012. Acne variants • Acneiform -

Keratosis Pilaris: a Common Follicular Hyperkeratosis
PEDIATRIC DERMATOLOGY Series Editor: Camila K. Janniger, MD Keratosis Pilaris: A Common Follicular Hyperkeratosis Sharon Hwang, MD; Robert A. Schwartz, MD, MPH Keratosis pilaris (KP) is a common inherited dis- 155 otherwise unaffected patients.2 In the adolescent order of follicular hyperkeratosis. It is character- population, its prevalence is postulated to be at least ized by small, folliculocentric keratotic papules 50%; it is more common in adolescent females than that may have surrounding erythema. The small males, seen in up to 80% of adolescent females.3 papules impart a stippled appearance to the skin The disorder is inherited in an autosomal domi- resembling gooseflesh. The disorder most com- nant fashion with variable penetrance; no specific monly affects the extensor aspects of the upper gene has been identified. In a study of 49 evaluated arms, upper legs, and buttocks. Patients with KP patients, there was a positive family history of KP in usually are asymptomatic, with complaints limited 19 patients (39%), while 27 patients (55%) had no to cosmetic appearance or mild pruritus. When family history of the disorder.4 diagnosing KP, the clinician should be aware that a number of diseases are associated with KP such Clinical Features as keratosis pilaris atrophicans, erythromelanosis The keratotic follicular papules of KP most commonly follicularis faciei et colli, and ichthyosis vulgaris. are grouped on the extensor aspects of the upper arms Treatment options vary, focusing on avoiding skin (Figure), upper legs, and buttocks.4 Other affected dryness, using emollients, and adding keratolytic locations may include the face and the trunk.5 The agents or topical steroids when necessary. -

Mallory Prelims 27/1/05 1:16 Pm Page I
Mallory Prelims 27/1/05 1:16 pm Page i Illustrated Manual of Pediatric Dermatology Mallory Prelims 27/1/05 1:16 pm Page ii Mallory Prelims 27/1/05 1:16 pm Page iii Illustrated Manual of Pediatric Dermatology Diagnosis and Management Susan Bayliss Mallory MD Professor of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine Director, Pediatric Dermatology St. Louis Children’s Hospital St. Louis, Missouri, USA Alanna Bree MD St. Louis University Director, Pediatric Dermatology Cardinal Glennon Children’s Hospital St. Louis, Missouri, USA Peggy Chern MD Department of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine St. Louis, Missouri, USA Mallory Prelims 27/1/05 1:16 pm Page iv © 2005 Taylor & Francis, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2005 by Taylor & Francis, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park Abingdon, Oxon OX14 4RN, UK Tel: +44 (0) 20 7017 6000 Fax: +44 (0) 20 7017 6699 Website: www.tandf.co.uk All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. Although every effort has been made to ensure that all owners of copyright material have been acknowledged in this publication, we would be glad to acknowledge in subsequent reprints or editions any omissions brought to our attention. -

Rush University Medical Center, May 2004
CHICAGO DERMATOLOGICAL SOCIETY RUSH UNIVERSITY MEDICAL CENTER CHICAGO, ILLINOIS MAY 19, 2004 TABLE OF CONTENTS Case # Title Page 1. Urticarial Vasculitis 1 2. Subcorneal Pustular Dermatosis 3 3. Animal-Type Melanoma 6 4. Multiple Clustered Dermatofibromas 8 5. Erythrokeratoderma Variabilis 10 6. “Unknown” 13 7. Anhidrotic Ectodermal Dysplasia 14 8. Linear Lichen Planus 17 9. Atypical Sweet’s Syndrome 19 10. Pancreatic Panniculitis 22 11. Bullous Sweet’s Syndrome 24 12. Plane Xanthoma 28 13. Hereditary Hemorrhagic Telangiectasia 31 14. Generalized Schamberg’s Disease 34 15. Eruptive Cherry Angiomas 37 16. Henoch-Schönlein Purpura 40 17. Generalized Granuloma Annulare 43 18. Thyroid Dermopathy 45 19. Lichen Spinulosus 47 20. Cystic Hygroma 49 Page 1 of 51 Case # 1 CHICAGO DERMATOLOGICAL SOCIETY RUSH UNIVERSITY MEDICAL CENTER CHICAGO, ILLINOIS MAY 19, 2004 CASE PRESENTED BY: Michael D. Tharp, M.D. and Alix J. Charles, M.D. History: This 51 year old white male was referred to our department for evaluation and treatment of a burning and pruritic erythematous eruption of seven months duration. The patient reported that lesions would appear on the trunk and extremities and last for more than twenty-four hours before resolving with hyperpigmentation. The eruption was also associated with swelling and joint pain of the hands. Treatment with zyrtec, allegra, clarinex, atarax, and medrol dose packs had been met with little success. Past Medical History: Family history of systemic lupus (daughter). Otherwise unremarkable. Physical Findings: The extremities, chest, and back display dozens of discrete and confluent erythematous and purpuric, non-blancheable macules and plaques. Some lesions have an annular configuration.