Primary Cutaneous Aggressive Epidermotropic Cytotoxic T-Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bexarotene (Targretin) Capsules Reference Number: PA.CP.PHAR.75 Effective Date: 09/11 Revision Log Last Review Date: 04/18
Clinical Policy: Bexarotene (Targretin) Capsules Reference Number: PA.CP.PHAR.75 Effective Date: 09/11 Revision Log Last Review Date: 04/18 Description The intent of the criteria is to ensure that patients follow selection elements established by Pennsylvania Health and Wellness ® clinical policy for bexarotene (Targretin®) capsules FDA Approved Indication(s) Targretin is indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma (CTCL) in patients who are refractory to at least one prior systemic therapy. Policy/Criteria It is the policy of Pennsylvania Health and Wellness that Targretin is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Cutaneous T-Cell Lymphoma (must meet all): 1. Diagnosis of cutaneous T-cell lymphoma (CTCL) (see Appendix B for CTCL subtypes); 2. Prescribed by or in consultation with an oncologist; 3. Age ≥ 18 years; 4. Dose does not exceed 400mg/m2 daily; Approval duration: 6 months B. Other diagnoses/indications: Refer to PA.CP.PHAR.57 - Global Biopharm Policy. II. Continued Approval A. Cutaneous T-Cell Lymphoma (must meet all): 1. Currently receiving medication via Pennsylvania Health and Wellness benefit or member has previously met all initial approval criteria; or the Continuity of Care policy (PA.LTSS.PHAR.01) applies; 2. Member is responding positively to therapy; 3. Dose does not exceed 400mg/m2 daily; Approval duration: 6months B. Other diagnoses/indications (must meet 1 or 2): 1. Currently receiving medication via Pennsylvania Health and Wellness benefit and documentation supports positive response to therapy; or the Continuity of Care policy (PA.LTSS.PHAR.01) applies; Approval duration: Duration of request or 6 months (whichever is less); or 2. -

Message from the President
DermNewsletter of the American OsteopathicLine College of Dermatology Spring 2016 Vol. 32, No. 1 Message from the President Greetings from Houston, Texas - again! As President of the AOCD, I welcome you to another edition of DermLine. As I think about our recent past and the dramatic changes afoot, I recall Suzanne Sirota Rozenberg, DO, FAOCD, Past President of AOCD, commenting in the Spring 2014 edition of DermLine about the coming AOA/ACGME merger. As I stated previously, “Today, at this moment, we are living in yesterday’s future.” Our future has arrived. Because the AOA, along with the Accreditation Council for Graduate Medical Education and the American Association of Colleges of Osteopathic Medicine, have agreed to a single accreditation system for graduate medical education programs in the United States, our graduates of osteopathic institutions, along with graduates of allopathic medical schools will complete their residency and/or fellowship education in ACGME-accredited programs and demonstrate achievement of common milestones and competencies side-by-side. I hope you realize the power in the previous statement. Trained together, osteopathic and allopathic graduates will no longer be divided. Perhaps the most exciting news to share is the AAD vote that just passed last month. This has been a battle we have been fighting for generations. The vote suffered defeat in 2004 and 2010. On both occasions, a majority of the membership voted in favor, but the required two-thirds majority required to approve a change to the bylaws fell short. I was told I would never see this vote go through in my lifetime. -

Atypical Variants of Mycosis Fungoides
Altınışık et al. Atypical Variants of Mycosis Fungoides REVIEW DOI: 10.4274/jtad.galenos.2021.09719 J Turk Acad Dermatol 2021;15(2):30-33 Atypical Variants of Mycosis Fungoides Dursun Dorukhan Altınışık, Özge Aşkın, Tuğba Kevser Uzunçakmak, Burhan Engin Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Dermatology, Istanbul, Turkey ABSTRACT Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL) and it is characterised by specific histological features. MF is in a spectrum of disorders which includes Sezary syndrome and other CTCL subtypes. Clinically and histopathologically it can imitate other T-cell mediated dermatosis hence making its diagnosis difficult. Due to different prognosis and difference in treatment strategies other subtypes of CTCL must be differentiated from MF. MF is a clinically indolent low-grade lymphoma which is seen in people aged between 55- 60 and it’s seen more commonly in men (2:1 ratio). Meanwhile other subtypes of MF like folliculotropic variant may show relatively a more aggressive course and our treatment strategy may differ. Keywords: Mycosis fungoides, Cutaneous T-cell lymphoma, Phototherapy, PUVA Introduction follicles frequently causing alopecia and in the paediatric population Mycosis fungoides (MF) is the most common type of cutaneous T-cell it tends to accompany hypopigmented patches and plaques. FMF lymphoma (CTCL) and it is characterised by specific histological preferentially involves the head and neck region however it can features [1]. MF is in a spectrum of disorders which includes Sezary sometimes involve the trunk or the extremities [6]. Patients with syndrome and other CTCL subtypes. Clinically and histopathologically FMF have significant pruritus which may be overlapped by S. -

Evaluation of Melanocyte Loss in Mycosis Fungoides Using SOX10 Immunohistochemistry
Article Evaluation of Melanocyte Loss in Mycosis Fungoides Using SOX10 Immunohistochemistry Cynthia Reyes Barron and Bruce R. Smoller * Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, NY 14642, USA; [email protected] * Correspondence: [email protected] Abstract: Mycosis fungoides (MF) is a subtype of primary cutaneous T-cell lymphoma (CTCL) with an indolent course that rarely progresses. Histologically, the lesions display a superficial lymphocytic infiltrate with epidermotropism of neoplastic T-cells. Hypopigmented MF is a rare variant that presents with hypopigmented lesions and is more likely to affect young patients. The etiology of the hypopigmentation is unclear. The aim of this study was to assess melanocyte loss in MF through immunohistochemistry (IHC) with SOX10. Twenty cases were evaluated, including seven of the hypopigmented subtype. The neoplastic epidermotropic infiltrate consisted predominantly of CD4+ T-cells in 65% of cases; CD8+ T-cells were present in moderate to abundant numbers in most cases. SOX10 IHC showed a decrease or focal complete loss of melanocytes in 50% of the cases. The predominant neoplastic cell type (CD4+/CD8+), age, race, gender, histologic features, and reported clinical pigmentation of the lesions were not predictive of melanocyte loss. A significant loss of melanocytes was observed in 43% of hypopigmented cases and 54% of conventional cases. Additional studies will increase our understanding of the relationship between observed pigmentation and the loss of melanocytes in MF. Citation: Barron, C.R.; Smoller, B.R. Evaluation of Melanocyte Loss in Keywords: mycosis fungoides; hypopigmented mycosis fungoides; SOX10; cutaneous T-cell lymphoma Mycosis Fungoides Using SOX10 Immunohistochemistry. -

Lichen Spinulosus: Case Report and Review of Literatures
Journal of Health Science 2015, 5(3A): 20-22 DOI: 10.5923/s.health.201501.07 Lichen Spinulosus: Case Report and Review of Literatures Khalid Al Hawsawi1,*, Kholood Almehmadi2, Bushra Alraddadi3, Ohood Aljuhani2 1Dermatology Consultant, Head of Dermatology Department, King Abdul Aziz Hospital, Makkah, Saudi Arabia 2Medical intern, King Abdul Aziz Hospital, Makkah, Saudi Arabia 3Dermatology Resident, King Abdul Aziz Hospital, Makkah, Saudi Arabia Abstract Lichen Spinulosus is a rare childhood disease. It is characterized by follicular hyperkeratotic papules that may coalesce into plaques. Individual lesion shows conical projection, hair like horny spine. The etiology is unknown but genetic predisposition has been suggested as a possible cause. It has a tendency to disappear spontaneously at puberty. Herein we present a 12-year-old boy presented with 6- month- history of persistent asymptomatic skin lesions. Skin examinations revealed multiple grouped scaly, hypopigmented tiny follicular papules with horny spines on his back. Soles showed planter keratoderma. Skin biopsy showed dilated hair follicle filled with keratin plugging. Diagnosis of LS associated with planter keratoderma was made. The patient responded well to topical lactic acid (12%), urea (20-30%), and betamethasone valerate (0.1%) preparations. Keywords Lichen spinulosa, Lichen Spinulosus 1. Introduction appearance. LS usually involves extensor surfaces of arms, trochanteric regions, neck, abdomen, buttocks, thigh, Lichen spinulosus (LS) is a genetic disorder of popliteal fossae, and knees. The histopathology of the lesion is not specific showing dilated hair follicle with keratin keratinization of hair follicles. LS was first described by Adamson in 1908. [1] It is a member of the family of plugging. -

Differentiating Keratosis Follicularis Spinulosa Decalvans from Monilithrix : Two Rare Causes of Scarring Alopecia in Young Male Siblings
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 18, Issue 5 Ser. 7 (May. 2019), PP 01-03 www.iosrjournals.org Differentiating Keratosis Follicularis Spinulosa Decalvans From Monilithrix : Two Rare Causes Of Scarring Alopecia In Young Male Siblings. 1 2 3 4 5 Heena Singdia , Rohit Garg , Sinni Jain , Vijay Paliwal , Puneet Bhargava , Deepak Mathur6 1,2,3,4,5,6(Department of dermatology, venereology and leprology, SMS Medical College, India) Corresponding Author: Heena Singdia Abstract: Keratosis follicularis spinulosa decalvans (KFSD) is a rare disorder affecting the hair follicles characterized by progressive cicatricial alopecia of the scalp and eyebrows, with usually X-linked recessive mode of transmission hence predominantly affecting Males.[1] Monilethrix is a rare structural Hair shaft disorder with autosomal dominant mode of transmission, characterized by short, fragile, brittle hair that breaks spontaneously resulting in patchy dystrophic alopecia.[2] Key words: Keratosis Follicularis Spinulosa Decalvans, Monilithrix, Hereditary Scarring Alopecias --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 02-05-2019 Date of acceptance: 16-05-2019 --------------------------------------------------------------------------------------------------------------------------------------------------- I. Introduction KFSD is a rare type of primary scarring alopecia with lymphocytic predominance. -

Primary Cutaneous Lymphoma: ESMO Clinical Recommendations for Diagnosis, Treatment and Follow-Up R
Annals of Oncology 19 (Supplement 2): ii72–ii76, 2008 clinical recommendations doi:10.1093/annonc/mdn095 Primary cutaneous lymphoma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up R. Dummer1 & M. Dreyling2 On behalf of the ESMO Guidelines Working Group* 1Department of Dermatology, University Hospital, Zu¨rich, Switzerland; 2Department of Medicine III, University Hospital Grosshadern, Munich, Germany introduction the quality of life due to their impact on skin appearance and annoying symptoms such as pruritus. In some cases they can Cutaneous lymphomas (CL) are the second most common already be disfiguring in early disease stages. In advanced stages, extranodal non-Hodgkin’s lymphomas. Their incidence is local skin problems are accompanied by systemic immune estimated at 1/100 000 yearly. Primary CL develop by definition suppression which results in an increased risk of infections and in the skin and remain confined to the skin for a long period of secondary malignancies. Some of the late-stage problems in time, while secondary CL reflect cutaneous spread from MF/SS patients might have been aggravated by earlier disseminated primary nodal or extranodal lymphomas. Primary therapeutic interventions, e.g. radiotherapy or phototherapy CL include a wide spectrum of clinically and histologically may contribute to mutations that increase the proliferative heterogeneous lymphoproliferative neoplasms: 75% of CL are and invasive capacity of the tumor cell populations. Cytotoxic cutaneous T-cell lymphomas (CTCL); 25% cutaneous B-cell drugs favor infectious complications. Most patients with lymphomas (CBCL); and a few percent other uncommon advanced disease may die due to secondary problems such as forms. CL and nodal or extracutaneous lymphomas with the infections. -

Treatment of Pagetoid Reticulosis with Intensity Modulated Radiation Therapy Charles Mount Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2014 Treatment of pagetoid reticulosis with intensity modulated radiation therapy Charles Mount Washington University School of Medicine in St. Louis Daniel Ferraro Washington University School of Medicine in St. Louis Alejandro Gru Washington University School of Medicine in St. Louis Matthew rB adbury Washington University School of Medicine in St. Louis Lynn Cornelius Washington University School of Medicine in St. Louis See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Mount, Charles; Ferraro, Daniel; Gru, Alejandro; Bradbury, Matthew; Cornelius, Lynn; and Gay, Hiram, ,"Treatment of pagetoid reticulosis with intensity modulated radiation therapy." Dermatology Online Journal.20,10. (2014). https://digitalcommons.wustl.edu/open_access_pubs/5149 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Authors Charles Mount, Daniel Ferraro, Alejandro Gru, Matthew Bradbury, Lynn Cornelius, and Hiram Gay This open access publication is available at Digital Commons@Becker: https://digitalcommons.wustl.edu/open_access_pubs/5149 Dermatology Online Journal UC Davis Peer Reviewed Title: Treatment of pagetoid reticulosis with intensity modulated radiation therapy Journal Issue: Dermatology Online Journal, 20(10) Author: Mount, Charles, Washington University in St. Louis Ferraro, Daniel, Washington University in St. Louis Gru, Alejandro, Washington University in St. Louis; The Ohio State University Bradbury, Matthew, Washington University in St. Louis Cornelius, Lynn, Washington University in St. -
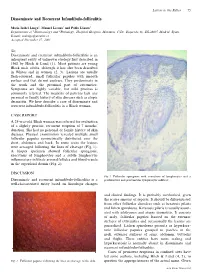
Disseminate and Recurrent Infundibulo-Folliculitis
Letters to the Editor 75 Disseminate and Recurrent Infundibulo-folliculitis Mar´õ a Isabel Longo1, Manuel Lecona2 and Pablo La´zaro1 Departments of 1Dermatology and 2Pathology, Hospital Grogorio Maran˜on, C/Dr. Esquerdo 46, ES-28007 Madrid, Spain. E-mail: [email protected] Accepted December 17, 2001. Sir, Disseminate and recurrent infundibulo-folliculitis is an infrequent entity of unknown etiology rst described in 1968 by Hitch & Lund (1). Most patients are young Black male adults, although it has also been described in Whites and in women (2, 3). Lesions are usually esh-coloured, small follicular papules with smooth surface and that do not coalesce. They predominate in the trunk and the proximal part of extremities. Symptoms are highly variable, but mild pruritus is commonly referred. The majority of patients lack any personal or family history of skin diseases such as atopic dermatitis. We here describe a case of disseminate and recurrent infundibulo-folliculitis in a Black woman. CASE REPORT A 25-year-old Black woman was referred for evaluation of a slightly pruritic, recurrent eruption of 7 months’ duration. She had no personal or family history of skin diseases. Physical examination revealed multiple small follicular papules symmetrically distributed over the chest, abdomen and back. In some areas the lesions were arranged following the lines of cleavage (Fig. 1). A biopsy specimen showed follicular spongiosis, exocytosis of lymphocytes and a subtle lymphocytic in ammatory in ltrate around follicles and blood vessels in the super cial dermis (Fig. 2). DISCUSSION Fig. 2. Follicular spongiosis with exocytosis of lymphocytes and a Disseminate and recurrent infundibulo-folliculitis is a perifollicular and perivascular lymphocytic in ltrate. -

Aafp Fmx 2020
Challenging Pediatric Rashes Richard P. Usatine, MD, FAAFP Professor, Family and Community Medicine Professor, Dermatology and Cutaneous Surgery University of Texas Health, San Antonio 1 ACTIVITY DISCLAIMER The material presented here is being made available by the American Academy of Family Physicians for educational purposes only. Please note that medical information is constantly changing; the information contained in this activity was accurate at the time of publication. This material is not intended to represent the only, nor necessarily best, methods or procedures appropriate for the medical situations discussed. Rather, it is intended to present an approach, view, statement, or opinion of the faculty, which may be helpful to others who face similar situations. The AAFP disclaims any and all liability for injury or other damages resulting to any individual using this material and for all claims that might arise out of the use of the techniques demonstrated therein by such individuals, whether these claims shall be asserted by a physician or any other person. Physicians may care to check specific details such as drug doses and contraindications, etc., in standard sources prior to clinical application. This material 2 might contain recommendations/guidelines developed by other organizations. Please note that although these guidelines might be included, this does not necessarily imply the endorsement by the AAFP. 2 1 Disclosure Statement It is the policy of the AAFP that all individuals in a position to control content disclose any relationships with commercial interests upon nomination/invitation of participation. Disclosure documents are reviewed for potential conflicts of interest. If conflicts are identified, they are resolved prior to confirmation of participation. -
Clinical and Trichoscopic Features in 18 Cases of Folliculotropic Mycosis Fungoides with Scalp Involvement
www.nature.com/scientificreports OPEN Clinical and trichoscopic features in 18 cases of Folliculotropic Mycosis Fungoides with scalp involvement Giuseppe Gallo1*, Alessandro Pileri2, Michela Starace2, Aurora Alessandrini2, Alba Guglielmo2, Simone Ribero1, Pietro Quaglino1 & Bianca Maria Piraccini2 Folliculotropic Mycosis Fungoides (FMF) is a rare variant of Mycosis Fungoides involving the scalp leading to alopecia. The clinical and trichoscopic features in 18 patients were analyzed and compared with the reports in the literature. Gender, age, disease stage, site of onset were taken into consideration. Clinical and trichoscopic analyses were performed on each patient. From a clinical point of view, Folliculotropic Mycosis Fungoides lesions involving the scalp presented as generalized alopecia (27.8%) or patchy-plaque alopecia (72.2%). Trichoscopic analysis revealed six most frequent features: single hair (83.3%), dotted dilated vessels (77.8%), broken-dystrophic hairs (66.7%), vellus hairs (61.1%), spermatozoa-like pattern vessels (55.6%), and yellow dots (55.6%). Additional identifed trichoscopic patterns were dilation of follicular openings, scales-crusts, purpuric dots, short hair with split-end, pigtail hairs, perifollicular hyperkeratosis, milky-white globules, black dots, white dots/lines and absence of follicular dots. These trichoscopic features were further correlated to clinical presentations and stage of the disease. The rarity of the disease is a limitation. The relatively high number of patients allowed to identify several clinical and trichoscopic patterns that could be featured as specifc or highly suspicious for FMF in order to consider trichoscopy as a complementary diagnostic approach and improve the diferential diagnoses between FMF and other scalp disorders. Mycosis Fungoides (MF) is the most common type of T-cell lymphoma and is characterized by small-medium atypical T lymphocytes infltrating the epidermis. -

Mycosis Fungoides and Sézary Syndrome: an Integrative Review of the Pathophysiology, Molecular Drivers, and Targeted Therapy
cancers Review Mycosis Fungoides and Sézary Syndrome: An Integrative Review of the Pathophysiology, Molecular Drivers, and Targeted Therapy Nuria García-Díaz 1 , Miguel Ángel Piris 2,† , Pablo Luis Ortiz-Romero 3,† and José Pedro Vaqué 1,*,† 1 Molecular Biology Department, Universidad de Cantabria—Instituto de Investigación Marqués de Valdecilla, IDIVAL, 39011 Santander, Spain; [email protected] 2 Department of Pathology, Fundación Jiménez Díaz, CIBERONC, 28040 Madrid, Spain; [email protected] 3 Department of Dermatology, Hospital 12 de Octubre, Institute i+12, CIBERONC, Medical School, University Complutense, 28041 Madrid, Spain; [email protected] * Correspondence: [email protected] † Same contribution. Simple Summary: In the last few years, the field of cutaneous T-cell lymphomas has experienced major advances. In the context of an active translational and clinical research field, next-generation sequencing data have boosted our understanding of the main molecular mechanisms that govern the biology of these entities, thus enabling the development of novel tools for diagnosis and specific therapy. Here, we focus on mycosis fungoides and Sézary syndrome; we review essential aspects of their pathophysiology, provide a rational mechanistic interpretation of the genomic data, and discuss the current and upcoming therapies, including the potential crosstalk between genomic alterations Citation: García-Díaz, N.; Piris, M.Á.; and the microenvironment, offering opportunities for targeted therapies. Ortiz-Romero, P.L.; Vaqué, J.P. Mycosis Fungoides and Sézary Abstract: Primary cutaneous T-cell lymphomas (CTCLs) constitute a heterogeneous group of diseases Syndrome: An Integrative Review of that affect the skin. Mycosis fungoides (MF) and Sézary syndrome (SS) account for the majority the Pathophysiology, Molecular of these lesions and have recently been the focus of extensive translational research.