Clinical and Trichoscopic Features in 18 Cases of Folliculotropic Mycosis Fungoides with Scalp Involvement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The End of the Queue: Hair As Symbol in Chinese History Michael Godley
East Asian History NUMBER 8 . DECEMBER 1994 THE CONTINUATION OF Paperson Far EasternHistory Institute of Advanced Studies Australian National University Editor Geremie R. Barme Assistant Editor Helen Lo Editorial Board John Clark Mark Elvin (Convenor) Helen Hardacre John Fincher Andrew Fraser Colin Jeffcott W. J. F. Jenner Lo Hui-min Gavan McCormack David Marr Tessa Morris-Suzuki Michael Underdown Business Manager Marion Weeks Production Helen Lo Design Maureen MacKenzie (Em Squared Typographic Design), Helen Lo Printed by Goanna Print, Fyshwick, ACT This is the eighth issue of East Asian History in the series previously entitled Papers on Far Eastern History. The journal is published twice a year. Contributions to The Editor, East Asian History Division of Pacific & Asian History, Research School of Pacific & Asian Studies Australian National University, Canberra ACT 0200, Australia Phone +61 62493140 Fax +61 62495525 Subscription Enquiries Subscription Manager, East Asian History, at the above address Annual Subscription Australia A$45 Overseas US$45 (for two issues) iii CONTENTS 1 Mid-Ch'ing New Text (Chin-wen) Classical Learning and its Han Provenance: the Dynamics of a Tradition of Ideas On-cho Ng 33 From Myth to Reality: Chinese Courtesans in Late-Qing Shanghai Christian Henriot 53 The End of the Queue: Hair as Symbol in Chinese History Michael Godley 73 Broken Journey: Nhfti Linh's "Going to France" Greg and Monique Lockhart 135 Chinese Masculinity: Theorising' Wen' and' Wu ' Kam Louie and Louise Edwards iv Cover calligraphy Yan Zhenqing �JU!iUruJ, Tang calligrapher and statesman Cover picture The walled city of Shanghai (Shanghai xianzhi, 1872) THE END OF THE QUEUE: HAIR AS SYMBOL IN CHINESE HISTORY ..J1! Michael R. -

A Randomized, Doubleblind, Placebo and Activecontrolled, Halfhead
BJD CONCISE COMMUNICATION British Journal of Dermatology A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata A. Trink,1 E. Sorbellini,1 P. Bezzola,1 L. Rodella,2 R. Rezzani,2 Y. Ramot3 and F. Rinaldi1 1International Hair Research Foundation (IHRF), Milan, Italy 2University of Brescia, Brescia, Italy 3Department of Dermatology, Hadassah – Hebrew University Medical Center, Jerusalem, Israel Summary Correspondence Background Alopecia areata (AA) is a common autoimmune condition, causing Fabio Rinaldi. inflammation-induced hair loss. This disease has very limited treatment possibili- E-mail: [email protected] ties, and no treatment is either curative or preventive. Platelet-rich plasma (PRP) has emerged as a new treatment modality in dermatology, and preliminary evi- Accepted for publication 16 April 2013 dence has suggested that it might have a beneficial role in hair growth. Objectives To evaluate the efficacy and safety of PRP for the treatment of AA in a Funding sources randomized, double-blind, placebo- and active-controlled, half-head, parallel- None. group study. Methods Forty-five patients with AA were randomized to receive intralesional Conflicts of interest injections of PRP, triamcinolone acetonide (TrA) or placebo on one half of None declared. their scalp. The other half was not treated. Three treatments were given for each DOI 10.1111/bjd.12397 patient, with intervals of 1 month. The endpoints were hair regrowth, hair dystrophy as measured by dermoscopy, burning or itching sensation, and cell proliferation as measured by Ki-67 evaluation. Patients were followed for 1 year. -

“Queue”: a Case of Chinese Scalping
Migration, Masculinity, and Mastering the “Queue”: A Case of Chinese Scalping RACHEL K. BRIGHT Keele University N 1906 a South African newspaper, The Prince, published a picture of Ia Chinese man’s scalp, which it had bought from an ex-prisoner. According to the original newspaper account and the subsequent government investigation, staff and prisoners were scalping Chinese men in the morgue on demand since at least May 1906, and selling them to colonial officials.1 ‘Queues’ were also being taken from living Chinese prisoners.2 The one sold to the newspaper was traced back to the execution of two Chinese prisoners at Pretoria Jail. When exhumed, both had been scalped. Prisoner witnesses attested that the 1 The Prince, 29 September 1906, 1116 had a photograph of the scalp (the front page), and 1118–1119 the story. See also 22 September 1906; 13 October 1906, 1166; Truth (Western Australia [WA]), 27 October 1906, 7; South African National Archives, Transvaal Foreign Labour Department (FLD) 7/147/20/20. Conclusions from Affidavits taken in connection with statements in The Prince regarding removal of a Chinaman’s Scalp; FLD7/ 147/20/20, Frank Oldrich Wheeler’s statement; FLD7/147/20/20, Alfred W. Sanders, District Surgeon, to Deputy Governor of Pretoria Prison, 9 October 1906; FLD7/147/20/20, Secretary, Law Department to Private Secretary, Acting Lieutenant-Governor, 16 November 1906; Warder Kidby’s statement; C. J. Hanrette, Director of Prisons, to Secretary of Law Department, 10 October 1906; FLD7/147/20/20, Secretary, Law Department, to Private Secretary, Acting Lieutenant-Governor, 16 November 1906;C. -

Download the Pigtail Contest Entry Form
Amanda Myers Board President Bratwurst Festival, Inc. Kevin Myers Festival Director On the streets of Beautiful Downtown Bucyrus, Ohio The Bratwurst Capital of America! Kylie Grau Contest Chairperson August 19th, 20th, & 21st, 2021 2021 Bratwurst Festival Pigtail Contest Sponsored by Tonya’s Hair Fashions The Pigtail Contest will take place Saturday, August 21, 2021 on the Schines Art Park Stage. The contest will start promptly at 12:00pm. Please take a few minutes to read the information carefully before entering your child into the contest. Please fill out the forms and mail or deliver with your $5.00 entry fee by check or money order to: Bratwurst Festival, Inc. Attn.: PIGTAIL 330 S. Sandusky Ave. Bucyrus, Ohio 44820 Pigtail Contest Information • Registration for the Pigtail Contest must be received by Monday, August 2, 2021 to guarantee a participation gift from the sponsor and Bratwurst Festival Pigtail T-Shirt Contest; however registration forms will be accepted through the end of the check-in time on the day of the contest. There is a $5.00 entry fee. • The Pigtail Contest is open to participants 3 years to 11 years of age. Age is determined as of August 1, 2021. • Check-in is required to be eligible to participate in the Pigtail Contest. Check in begins at 11:15 a.m. and concludes at 11:50 a.m. Even if the contestant pre-registered, they still are required to check-in. o NOTE- check in will allow for every contestant to be accounted for and placed in the appropriate age groups with the proper name tag. -

Atypical Variants of Mycosis Fungoides
Altınışık et al. Atypical Variants of Mycosis Fungoides REVIEW DOI: 10.4274/jtad.galenos.2021.09719 J Turk Acad Dermatol 2021;15(2):30-33 Atypical Variants of Mycosis Fungoides Dursun Dorukhan Altınışık, Özge Aşkın, Tuğba Kevser Uzunçakmak, Burhan Engin Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Dermatology, Istanbul, Turkey ABSTRACT Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL) and it is characterised by specific histological features. MF is in a spectrum of disorders which includes Sezary syndrome and other CTCL subtypes. Clinically and histopathologically it can imitate other T-cell mediated dermatosis hence making its diagnosis difficult. Due to different prognosis and difference in treatment strategies other subtypes of CTCL must be differentiated from MF. MF is a clinically indolent low-grade lymphoma which is seen in people aged between 55- 60 and it’s seen more commonly in men (2:1 ratio). Meanwhile other subtypes of MF like folliculotropic variant may show relatively a more aggressive course and our treatment strategy may differ. Keywords: Mycosis fungoides, Cutaneous T-cell lymphoma, Phototherapy, PUVA Introduction follicles frequently causing alopecia and in the paediatric population Mycosis fungoides (MF) is the most common type of cutaneous T-cell it tends to accompany hypopigmented patches and plaques. FMF lymphoma (CTCL) and it is characterised by specific histological preferentially involves the head and neck region however it can features [1]. MF is in a spectrum of disorders which includes Sezary sometimes involve the trunk or the extremities [6]. Patients with syndrome and other CTCL subtypes. Clinically and histopathologically FMF have significant pruritus which may be overlapped by S. -

Symbolism of Hairstyles in Korea and Japan Research Material
RESEARCH MATERIAL Na-Young Choi Wonkwang University, South Korea Symbolism of Hairstyles in Korea and Japan Abstract The paper attempts to examine the origins and changes in the hairstyles of Korea and Japan from ancient to early modern times and to compare their features in order to determine what they have in common. The results can be summarized in four points: First, hairstyles were thought to fend off evil influences; second, they were a means to express an ideal of beauty; third, they were an expression of a woman’s marital status; and fourth, they were an expression of social status and wealth. Keywords: hairstyles—symbolism—magical meaning—standard of beauty Asian Folklore Studies, Volume 65, 2006: 69–86 his paper seeks to examine and describe women’s hairstyle changes in Korea and Japan, which belong to the same cultural zone of East Asia, from ancient to early modern times. These countries are in a monsoon zone,T they were originally agricultural societies, and they actively engaged in cultural exchange from earliest times, a factor that is of importance to the fol- lowing discussion. Hairdressing, which varied according to clothing styles, was primarily used to express one’s position, nature, and sensibility rather than to put one’s hair in order. Hairstyles also differed according to the ethnic back- ground, natural features of a person, and beauty standards of a particular peri- od: they revealed one’s nationality, sex, age, occupation, and religion. While previous research on hairstyles in Korea focused on the changes in Korean hairstyles based on historical periods, the hairstyles of Korea and Japan have not been compared to examine their common symbolism, such as the implication of magical meanings, expression of beauty, symbol of marital status, and indication of social position and wealth. -

Lichen Spinulosus: Case Report and Review of Literatures
Journal of Health Science 2015, 5(3A): 20-22 DOI: 10.5923/s.health.201501.07 Lichen Spinulosus: Case Report and Review of Literatures Khalid Al Hawsawi1,*, Kholood Almehmadi2, Bushra Alraddadi3, Ohood Aljuhani2 1Dermatology Consultant, Head of Dermatology Department, King Abdul Aziz Hospital, Makkah, Saudi Arabia 2Medical intern, King Abdul Aziz Hospital, Makkah, Saudi Arabia 3Dermatology Resident, King Abdul Aziz Hospital, Makkah, Saudi Arabia Abstract Lichen Spinulosus is a rare childhood disease. It is characterized by follicular hyperkeratotic papules that may coalesce into plaques. Individual lesion shows conical projection, hair like horny spine. The etiology is unknown but genetic predisposition has been suggested as a possible cause. It has a tendency to disappear spontaneously at puberty. Herein we present a 12-year-old boy presented with 6- month- history of persistent asymptomatic skin lesions. Skin examinations revealed multiple grouped scaly, hypopigmented tiny follicular papules with horny spines on his back. Soles showed planter keratoderma. Skin biopsy showed dilated hair follicle filled with keratin plugging. Diagnosis of LS associated with planter keratoderma was made. The patient responded well to topical lactic acid (12%), urea (20-30%), and betamethasone valerate (0.1%) preparations. Keywords Lichen spinulosa, Lichen Spinulosus 1. Introduction appearance. LS usually involves extensor surfaces of arms, trochanteric regions, neck, abdomen, buttocks, thigh, Lichen spinulosus (LS) is a genetic disorder of popliteal fossae, and knees. The histopathology of the lesion is not specific showing dilated hair follicle with keratin keratinization of hair follicles. LS was first described by Adamson in 1908. [1] It is a member of the family of plugging. -

Differentiating Keratosis Follicularis Spinulosa Decalvans from Monilithrix : Two Rare Causes of Scarring Alopecia in Young Male Siblings
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 18, Issue 5 Ser. 7 (May. 2019), PP 01-03 www.iosrjournals.org Differentiating Keratosis Follicularis Spinulosa Decalvans From Monilithrix : Two Rare Causes Of Scarring Alopecia In Young Male Siblings. 1 2 3 4 5 Heena Singdia , Rohit Garg , Sinni Jain , Vijay Paliwal , Puneet Bhargava , Deepak Mathur6 1,2,3,4,5,6(Department of dermatology, venereology and leprology, SMS Medical College, India) Corresponding Author: Heena Singdia Abstract: Keratosis follicularis spinulosa decalvans (KFSD) is a rare disorder affecting the hair follicles characterized by progressive cicatricial alopecia of the scalp and eyebrows, with usually X-linked recessive mode of transmission hence predominantly affecting Males.[1] Monilethrix is a rare structural Hair shaft disorder with autosomal dominant mode of transmission, characterized by short, fragile, brittle hair that breaks spontaneously resulting in patchy dystrophic alopecia.[2] Key words: Keratosis Follicularis Spinulosa Decalvans, Monilithrix, Hereditary Scarring Alopecias --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 02-05-2019 Date of acceptance: 16-05-2019 --------------------------------------------------------------------------------------------------------------------------------------------------- I. Introduction KFSD is a rare type of primary scarring alopecia with lymphocytic predominance. -

The Ming Dynasty
The East Asian World 1400–1800 Key Events As you read this chapter, look for the key events in the history of the East Asian world. • China closed its doors to the Europeans during the period of exploration between 1500 and 1800. • The Ming and Qing dynasties produced blue-and-white porcelain and new literary forms. • Emperor Yong Le began renovations on the Imperial City, which was expanded by succeeding emperors. The Impact Today The events that occurred during this time still impact our lives today. • China today exports more goods than it imports. • Chinese porcelain is collected and admired throughout the world. • The Forbidden City in China is an architectural wonder that continues to attract people from around the world. • Relations with China today still require diplomacy and skill. World History Video The Chapter 16 video, “The Samurai,” chronicles the role of the warrior class in Japanese history. 1514 Portuguese arrive in China Chinese sailing ship 1400 1435 1470 1505 1540 1575 1405 1550 Zheng He Ming dynasty begins voyages flourishes of exploration Ming dynasty porcelain bowl 482 Art or Photo here The Forbidden City in the heart of Beijing contains hundreds of buildings. 1796 1598 1644 1750 White Lotus HISTORY Japanese Last Ming Edo is one of rebellion unification emperor the world’s weakens Qing begins dies largest cities dynasty Chapter Overview Visit the Glencoe World History Web site at 1610 1645 1680 1715 1750 1785 tx.wh.glencoe.com and click on Chapter 16–Chapter Overview to preview chapter information. 1603 1661 1793 Tokugawa Emperor Britain’s King rule begins Kangxi begins George III sends “Great 61-year reign trade mission Peace” to China Japanese samurai 483 Emperor Qianlong The meeting of Emperor Qianlong and Lord George Macartney Mission to China n 1793, a British official named Lord George Macartney led a mission on behalf of King George III to China. -
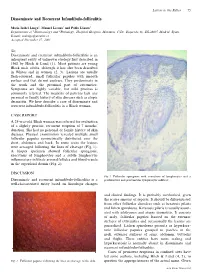
Disseminate and Recurrent Infundibulo-Folliculitis
Letters to the Editor 75 Disseminate and Recurrent Infundibulo-folliculitis Mar´õ a Isabel Longo1, Manuel Lecona2 and Pablo La´zaro1 Departments of 1Dermatology and 2Pathology, Hospital Grogorio Maran˜on, C/Dr. Esquerdo 46, ES-28007 Madrid, Spain. E-mail: [email protected] Accepted December 17, 2001. Sir, Disseminate and recurrent infundibulo-folliculitis is an infrequent entity of unknown etiology rst described in 1968 by Hitch & Lund (1). Most patients are young Black male adults, although it has also been described in Whites and in women (2, 3). Lesions are usually esh-coloured, small follicular papules with smooth surface and that do not coalesce. They predominate in the trunk and the proximal part of extremities. Symptoms are highly variable, but mild pruritus is commonly referred. The majority of patients lack any personal or family history of skin diseases such as atopic dermatitis. We here describe a case of disseminate and recurrent infundibulo-folliculitis in a Black woman. CASE REPORT A 25-year-old Black woman was referred for evaluation of a slightly pruritic, recurrent eruption of 7 months’ duration. She had no personal or family history of skin diseases. Physical examination revealed multiple small follicular papules symmetrically distributed over the chest, abdomen and back. In some areas the lesions were arranged following the lines of cleavage (Fig. 1). A biopsy specimen showed follicular spongiosis, exocytosis of lymphocytes and a subtle lymphocytic in ammatory in ltrate around follicles and blood vessels in the super cial dermis (Fig. 2). DISCUSSION Fig. 2. Follicular spongiosis with exocytosis of lymphocytes and a Disseminate and recurrent infundibulo-folliculitis is a perifollicular and perivascular lymphocytic in ltrate. -

Aafp Fmx 2020
Challenging Pediatric Rashes Richard P. Usatine, MD, FAAFP Professor, Family and Community Medicine Professor, Dermatology and Cutaneous Surgery University of Texas Health, San Antonio 1 ACTIVITY DISCLAIMER The material presented here is being made available by the American Academy of Family Physicians for educational purposes only. Please note that medical information is constantly changing; the information contained in this activity was accurate at the time of publication. This material is not intended to represent the only, nor necessarily best, methods or procedures appropriate for the medical situations discussed. Rather, it is intended to present an approach, view, statement, or opinion of the faculty, which may be helpful to others who face similar situations. The AAFP disclaims any and all liability for injury or other damages resulting to any individual using this material and for all claims that might arise out of the use of the techniques demonstrated therein by such individuals, whether these claims shall be asserted by a physician or any other person. Physicians may care to check specific details such as drug doses and contraindications, etc., in standard sources prior to clinical application. This material 2 might contain recommendations/guidelines developed by other organizations. Please note that although these guidelines might be included, this does not necessarily imply the endorsement by the AAFP. 2 1 Disclosure Statement It is the policy of the AAFP that all individuals in a position to control content disclose any relationships with commercial interests upon nomination/invitation of participation. Disclosure documents are reviewed for potential conflicts of interest. If conflicts are identified, they are resolved prior to confirmation of participation. -
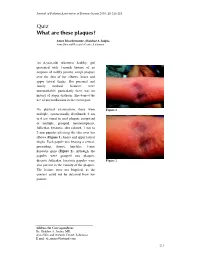
Quiz What Are These Piaques?
Journal of Pakistan Association of Dermatologists 2003; 13 : 211-213. Quiz What are these pIaques? Amor Khachemoune, Shahbaz A Janjua Ayza Skin and Research Centre , Lalamusa An 8-year-old, otherwise healthy, girl presented with 1-month history of an eruption of mildly pruritic, rough plaques over the skin of her elbows, knees and upper lateral thighs. Her personal and family medical histories were unremarkable; particularly there was no history of atopic diathesis. She denied the use of any medication in the recent past. On physical examination, there were Figure 1 multiple, symmetrically distributed, 2 cm to 6 cm round to oval plaques comprised of multiple, grouped, monomorphous, follicular, keratotic, skin colored, 1 mm to 2 mm papules affecting the skin over her elbows ( Figure 1 ), knees and upper lateral thighs. Each papule was bearing a central, protruding, thorny, hair-like, 1-mm keratotic spine ( Figure 2 ). Although, the papules were grouped into plaques, discrete follicular, keratotic papules were Figure 2 also present in the vicinity of the plaques. The lesions were not biopsied, as the consent could not be obtained from her parents. Address for Correspondence Dr. Shahbaz A. Janjua, MD, Ayza Skin and research Centre, Lalamusa. E mail: [email protected] 211 What are these plaques? Amor Khachemoune & S.A Janjua . Diagnosis On histopathology, LS lesions show a Lichen spinulosus central, infundibular, keratotic plug dilating the follicle and protruding above Lichen spinulosus (LS) is a rare, the epidermal surface. Follicular idiopathic, follicular, keratotic dermatosis hyperkeratosis, parakeratosis and occurring mostly in children, adolescents acanthosis may be present. Dermal and young adults.