Northwestern University, October 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Keratosis Circumscripta Revisited: a Case Report and Review of the Literature
CONTINUING MEDICAL EDUCATION Keratosis Circumscripta Revisited: A Case Report and Review of the Literature LCDR Eric P. Brumwell, MC, USN; LCDR Sean J. Murphy, MC, USN GOAL To understand keratosis circumscripta to better manage patients with the condition OBJECTIVES Upon completion of this activity, dermatologists and general practitioners should be able to: 1. Describe the clinical presentation of keratosis circumscripta. 2. Discuss the disorders often compared to keratosis circumscripta. 3. Identify treatment options for keratosis circumscripta. CME Test on page 378. This article has been peer reviewed and approved Einstein College of Medicine is accredited by by Michael Fisher, MD, Professor of Medicine, the ACCME to provide continuing medical edu- Albert Einstein College of Medicine. Review date: cation for physicians. April 2007. Albert Einstein College of Medicine designates This activity has been planned and imple- this educational activity for a maximum of 1 AMA mented in accordance with the Essential Areas PRA Category 1 CreditTM. Physicians should only and Policies of the Accreditation Council for claim credit commensurate with the extent of their Continuing Medical Education through the participation in the activity. joint sponsorship of Albert Einstein College of This activity has been planned and produced in Medicine and Quadrant HealthCom, Inc. Albert accordance with ACCME Essentials. Drs. Brumwell and Murphy report no conflict of interest. The authors report no discussion of off-label use. Dr. Fisher reports no conflict of interest. Keratosis circumscripta, also known as psoriasis been shown to improve, but not resolve, with kerat- circumscripta with palmoplantar keratosis, is a inolytic therapies. Some debate exists concern- rarely reported condition that manifests as well- ing the terminology used to identify this condition. -

Mal De Meleda in a Taiwanese
S.C. Chao, F.J. Lai, M.H. Yang, et al MAL DE MELEDA IN A TAIWANESE Sheau-Chiou Chao, Feng-Jei Lai, Mei-Hui Yang, and Julia Yu-Yun Lee Abstract: Mal de Meleda (MDM) is a rare form of recessive transgressive palmoplantar erythrokeratoderma for which mutations in the ARS gene have been identified recently. The ARS gene encodes SLURP-1, a secreted epidermal neuromodulator involved in epidermal homeostasis and inhibition of tumor necrosis factor-α release. A 27-year- old Taiwanese woman who had a history of palmoplantar keratoderma since birth presented with severe erythrokeratoderma of the hands and feet in a glove-and-stocking distribution with conical tapering of the fingers, and involvement of the skin over the major joints and thighs. There were also widespread mottled hyperpigmented macules. Mutation analysis revealed a homozygous missense mutation (G86R) in exon 3 of ARS gene of this patient. Key words: ARS protein, human; Keratoderma, palmoplantar; Mutation, missense; Neuronal nicotinic acetylcholine receptor alpha7; Taiwan J Formos Med Assoc 2005;104:276-8 Keratoderma palmoplantare transgrediens or mal de that appeared soon after birth. The family reported no Meleda (MDM) is a rare autosomal recessive inflam- known consanguinity. She was the only affected member matory keratoderma, characterized by diffuse erythema in the family. On examination, the patient had marked and hyperkeratosis of the hands and feet that appears erythema and hyperkeratosis of the hands and feet soon after birth and progressively extends to the dorsal in a glove-and-stocking distribution, accompanied by aspect of the hands and feet and around the wrist malodor (Fig.). -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -

Atypical Variants of Mycosis Fungoides
Altınışık et al. Atypical Variants of Mycosis Fungoides REVIEW DOI: 10.4274/jtad.galenos.2021.09719 J Turk Acad Dermatol 2021;15(2):30-33 Atypical Variants of Mycosis Fungoides Dursun Dorukhan Altınışık, Özge Aşkın, Tuğba Kevser Uzunçakmak, Burhan Engin Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Dermatology, Istanbul, Turkey ABSTRACT Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL) and it is characterised by specific histological features. MF is in a spectrum of disorders which includes Sezary syndrome and other CTCL subtypes. Clinically and histopathologically it can imitate other T-cell mediated dermatosis hence making its diagnosis difficult. Due to different prognosis and difference in treatment strategies other subtypes of CTCL must be differentiated from MF. MF is a clinically indolent low-grade lymphoma which is seen in people aged between 55- 60 and it’s seen more commonly in men (2:1 ratio). Meanwhile other subtypes of MF like folliculotropic variant may show relatively a more aggressive course and our treatment strategy may differ. Keywords: Mycosis fungoides, Cutaneous T-cell lymphoma, Phototherapy, PUVA Introduction follicles frequently causing alopecia and in the paediatric population Mycosis fungoides (MF) is the most common type of cutaneous T-cell it tends to accompany hypopigmented patches and plaques. FMF lymphoma (CTCL) and it is characterised by specific histological preferentially involves the head and neck region however it can features [1]. MF is in a spectrum of disorders which includes Sezary sometimes involve the trunk or the extremities [6]. Patients with syndrome and other CTCL subtypes. Clinically and histopathologically FMF have significant pruritus which may be overlapped by S. -

The First Reported Case of a Variant of Mal De Maleda of the Gamborg
Our Dermatology Online Case Report Th e fi rrstst rreportedeported ccasease ooff a vvariantariant ooff MMalal ddee MMaledaaleda ooff tthehe GGamborg-Nielsenamborg-Nielsen ttypeype iinn aann EEgyptiangyptian ooriginrigin ppatientatient Khalid M. Al-Husain1, Ahmed A. Al-Thubaiti1, Farah M. Alzahrani2, Iqbal A. Bukhari3, Mohammad El-Shawarby4 1College of Medicine, Imam Abdulrahman Bin Faisal University (IAU), Dammam, Kingdom of Saudi Arabia, 2College of Medicine, Arabian Gulf University (AGU), Bahrain, 3Dermatology Department, College of Medicine, Imam Abdulrahman Bin Faisal University (IAU) and King Fahd Hospital of the University, Dammam, Kingdom of Saudi Arabia, 4Pathology Department, College of Medicine, Imam Abdulrahman Bin Faisal University (IAU) and King Fahd Hospital of the University, Dammam, Kingdom of Saudi Arabia Corresponding author: Prof. Iqbal A. Bukhari, E-mail: [email protected] ABSTRACT Mal de Meleda is a rare genodermatosis with an autosomal recessive inheritance. Mutations in the SLURP1 gene are the cause of this disease. Clinically, it is characterized by progressive palmoplantar hyperkeratosis exhibiting a transgradiens pattern extending to the dorsal aspects of the hands and feet in a glove and stocking pattern. It is also associated with hyperhidrosis, nail changes, subungual hyperkeratosis and perioral erythema. Here we report the first case of Gamborg-Nielsen variant of Mal de Meleda disorder in a patient of an Egyptian origin. Key words: Mal de Meleda; Keratoderma; Genodermatosis; SLURP1 gene INTRODUCTION in -

Lichen Spinulosus: Case Report and Review of Literatures
Journal of Health Science 2015, 5(3A): 20-22 DOI: 10.5923/s.health.201501.07 Lichen Spinulosus: Case Report and Review of Literatures Khalid Al Hawsawi1,*, Kholood Almehmadi2, Bushra Alraddadi3, Ohood Aljuhani2 1Dermatology Consultant, Head of Dermatology Department, King Abdul Aziz Hospital, Makkah, Saudi Arabia 2Medical intern, King Abdul Aziz Hospital, Makkah, Saudi Arabia 3Dermatology Resident, King Abdul Aziz Hospital, Makkah, Saudi Arabia Abstract Lichen Spinulosus is a rare childhood disease. It is characterized by follicular hyperkeratotic papules that may coalesce into plaques. Individual lesion shows conical projection, hair like horny spine. The etiology is unknown but genetic predisposition has been suggested as a possible cause. It has a tendency to disappear spontaneously at puberty. Herein we present a 12-year-old boy presented with 6- month- history of persistent asymptomatic skin lesions. Skin examinations revealed multiple grouped scaly, hypopigmented tiny follicular papules with horny spines on his back. Soles showed planter keratoderma. Skin biopsy showed dilated hair follicle filled with keratin plugging. Diagnosis of LS associated with planter keratoderma was made. The patient responded well to topical lactic acid (12%), urea (20-30%), and betamethasone valerate (0.1%) preparations. Keywords Lichen spinulosa, Lichen Spinulosus 1. Introduction appearance. LS usually involves extensor surfaces of arms, trochanteric regions, neck, abdomen, buttocks, thigh, Lichen spinulosus (LS) is a genetic disorder of popliteal fossae, and knees. The histopathology of the lesion is not specific showing dilated hair follicle with keratin keratinization of hair follicles. LS was first described by Adamson in 1908. [1] It is a member of the family of plugging. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008. -

Differentiating Keratosis Follicularis Spinulosa Decalvans from Monilithrix : Two Rare Causes of Scarring Alopecia in Young Male Siblings
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 18, Issue 5 Ser. 7 (May. 2019), PP 01-03 www.iosrjournals.org Differentiating Keratosis Follicularis Spinulosa Decalvans From Monilithrix : Two Rare Causes Of Scarring Alopecia In Young Male Siblings. 1 2 3 4 5 Heena Singdia , Rohit Garg , Sinni Jain , Vijay Paliwal , Puneet Bhargava , Deepak Mathur6 1,2,3,4,5,6(Department of dermatology, venereology and leprology, SMS Medical College, India) Corresponding Author: Heena Singdia Abstract: Keratosis follicularis spinulosa decalvans (KFSD) is a rare disorder affecting the hair follicles characterized by progressive cicatricial alopecia of the scalp and eyebrows, with usually X-linked recessive mode of transmission hence predominantly affecting Males.[1] Monilethrix is a rare structural Hair shaft disorder with autosomal dominant mode of transmission, characterized by short, fragile, brittle hair that breaks spontaneously resulting in patchy dystrophic alopecia.[2] Key words: Keratosis Follicularis Spinulosa Decalvans, Monilithrix, Hereditary Scarring Alopecias --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 02-05-2019 Date of acceptance: 16-05-2019 --------------------------------------------------------------------------------------------------------------------------------------------------- I. Introduction KFSD is a rare type of primary scarring alopecia with lymphocytic predominance. -

Palmoplantar Keratoderma of the Gamborg-Nielsen Type Is Caused by Mutations in the SLURP1 Gene and Represents a Variant of Mal De Meleda
Acta Derm Venereol 2014; 94: 707–710 CLINICAL REPORT Palmoplantar Keratoderma of the Gamborg-Nielsen Type is Caused by Mutations in the SLURP1 Gene and Represents a Variant of Mal de Meleda Linshu ZHAO1, Anders VAHLQUIST2*, Marie VIRtanen2, Lena WENNERSTRAND3, Lisbet K. LIND3, Anita LUNDSTRÖM4 and Maritta HELLSTRÖM PIGG1 Departments of 1Immunology, Genetics and Pathology and 2Medical Sciences, Uppsala University, Uppsala, and 3Departments of Medical Biosciences, Medical and Clinical Genetics and 4Public Health and Clinical Medicine, Umeå University, Umeå, Sweden Palmoplantar keratoderma of the Gamborg-Nielsen also observed. Mal de Meleda (MDM; OMIM 248300) type (PPK-GN) is a rare autosomal recessive skin disor- or keratosis palmoplantaris transgradiens of Siemens der described in patients from Sweden. Mal de Meleda is an autosomal recessive skin disorder first described (MDM) is also a rare autosomal recessive inherited PPK in 1898 by Neumann (2) in patients from the island of first reported in 5 families from the island of Meleda. Mljet (Meleda) in Dalmatia, Croatia. MDM is clinically The 2 conditions phenotypically overlap and are charac- characterised by symmetric transgressive PPK, and so- terised by palmoplantar erythematous hyperkeratotic metimes associated with lichenoid or keratotic plaques plaques. The genetic background giving rise to PPK-GN over joints, redness in the palms and soles, brachydactyly, has hitherto been unknown, whereas MDM is known to cone-shaped fingers, pseudoainhum and nail abnorma- be caused by mutations in the gene encoding secreted lities with pachyonychia. The progressive lesions can Ly-6/uPAR-related protein 1, SLURP-1. In the present lead to reduced mobility of hands and feet because of study we scrutinised individuals affected by PPK-GN for contractions (2–8). -
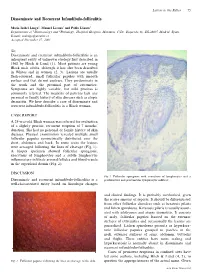
Disseminate and Recurrent Infundibulo-Folliculitis
Letters to the Editor 75 Disseminate and Recurrent Infundibulo-folliculitis Mar´õ a Isabel Longo1, Manuel Lecona2 and Pablo La´zaro1 Departments of 1Dermatology and 2Pathology, Hospital Grogorio Maran˜on, C/Dr. Esquerdo 46, ES-28007 Madrid, Spain. E-mail: [email protected] Accepted December 17, 2001. Sir, Disseminate and recurrent infundibulo-folliculitis is an infrequent entity of unknown etiology rst described in 1968 by Hitch & Lund (1). Most patients are young Black male adults, although it has also been described in Whites and in women (2, 3). Lesions are usually esh-coloured, small follicular papules with smooth surface and that do not coalesce. They predominate in the trunk and the proximal part of extremities. Symptoms are highly variable, but mild pruritus is commonly referred. The majority of patients lack any personal or family history of skin diseases such as atopic dermatitis. We here describe a case of disseminate and recurrent infundibulo-folliculitis in a Black woman. CASE REPORT A 25-year-old Black woman was referred for evaluation of a slightly pruritic, recurrent eruption of 7 months’ duration. She had no personal or family history of skin diseases. Physical examination revealed multiple small follicular papules symmetrically distributed over the chest, abdomen and back. In some areas the lesions were arranged following the lines of cleavage (Fig. 1). A biopsy specimen showed follicular spongiosis, exocytosis of lymphocytes and a subtle lymphocytic in ammatory in ltrate around follicles and blood vessels in the super cial dermis (Fig. 2). DISCUSSION Fig. 2. Follicular spongiosis with exocytosis of lymphocytes and a Disseminate and recurrent infundibulo-folliculitis is a perifollicular and perivascular lymphocytic in ltrate. -

Aafp Fmx 2020
Challenging Pediatric Rashes Richard P. Usatine, MD, FAAFP Professor, Family and Community Medicine Professor, Dermatology and Cutaneous Surgery University of Texas Health, San Antonio 1 ACTIVITY DISCLAIMER The material presented here is being made available by the American Academy of Family Physicians for educational purposes only. Please note that medical information is constantly changing; the information contained in this activity was accurate at the time of publication. This material is not intended to represent the only, nor necessarily best, methods or procedures appropriate for the medical situations discussed. Rather, it is intended to present an approach, view, statement, or opinion of the faculty, which may be helpful to others who face similar situations. The AAFP disclaims any and all liability for injury or other damages resulting to any individual using this material and for all claims that might arise out of the use of the techniques demonstrated therein by such individuals, whether these claims shall be asserted by a physician or any other person. Physicians may care to check specific details such as drug doses and contraindications, etc., in standard sources prior to clinical application. This material 2 might contain recommendations/guidelines developed by other organizations. Please note that although these guidelines might be included, this does not necessarily imply the endorsement by the AAFP. 2 1 Disclosure Statement It is the policy of the AAFP that all individuals in a position to control content disclose any relationships with commercial interests upon nomination/invitation of participation. Disclosure documents are reviewed for potential conflicts of interest. If conflicts are identified, they are resolved prior to confirmation of participation. -

Towards a Comprehensive Resource for Elucidating the Pathogenesis of Inherited Keratodermas
Towards a Comprehensive Resource for Elucidating the Pathogenesis of Inherited Keratodermas Dr Mozheh Zamiri BSc (Hons), MB ChB, MRCP Doctorate of Medicine University of Edinburgh 2009 Alan Lyell Centre for Dermatology, Glasgow & Section of Dermatology, University of Glasgow ABSTRACT Keratoderma – pathological hyperkeratosis of palms and soles - is a cause of disability in many clinical situations, including the rare and heterogeneous group of inherited palmoplantar keratodermas (PPKs). The aim of this study was to work towards better understanding of molecular mechanisms active in the pathogenesis of PPK by the creation of a cell and tissue culture resource and its initial application to laboratory studies. My study was based on a diverse group of autosomal dominant disorders, previously ascertained in families from Scotland, in whom the precise genetic aetiology was known. I established a tissue and cell culture resource of inherited keratodermas of known single-gene aetiology from patients with proven keratin 1, 9, 17, loricrin and mitochondrial mutations. An additional pedigree with striate keratoderma with an unknown mutation was recruited, and the causative mutation identified as a novel heterozygous A-to-T transversion in exon 5 (c.430A>T) of the desmoglein 1 gene, converting an arginine residue to a premature termination codon (p.Arg144stop). The keratinocyte culture resource was established from patients with keratin 1, 9, 17 and loricrin mutations, as well as controls. Due to the pain associated with direct infiltration of plantar skin, biopsies were obtained using peripheral nerve block for plantar biopsy. The effectiveness of this approach, which may be useful for future administration of treatment, was made the subject of an open clinical trial.