Modeling Leigh Syndrome Using Patient-Specific Induced Pluripotent Stem Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BR27069-Stemdiff™ Cerebral Organoid
CEREBRAL ORGANOIDS STEMdiff™ Cerebral Organoid Kit Scientists Helping Scientists™ | WWW.STEMCELL.COM TABLE OF CONTENTS 3 Introduction 4 Cerebral Organoid Formation 5 Cerebral Organoid Characterization 7 Performance of STEMdiffTM Cerebral Organoid Kit vs. Unqualified Reagents 7 Product Information 7 References Introduction For the Culture and Maturation of Why Use the STEMdiff™ Cerebral Organoid Kit? Human Cerebral Organoids PHYSIOLOGICAL. Three-dimensional in vitro system The metazoan brain is a highly complex and organized structure. recapitulates the developmental processes and Two-dimensional (2D) neural cultures derived from human organization of the developing brain. pluripotent stem cells (hPSCs) are useful models to study the nervous INNOVATIVE. Serum-free human pluripotent stem system, but they are limited in their capacity to recapitulate the cell-based model enables the study of development and complex organization of brain tissues. disease processes. hPSC-derived cerebral organoids are three-dimensional (3D) in vitro OPTIMIZED. Formulation is optimized for increased culture systems that recapitulate the developmental processes and efficiency of organoid formation. organization of the developing human brain. They provide a physiologically relevant in vitro model for the RELIABLE. Rigorous raw material screening and study of neurological development and disease processes that extensive quality control testing ensure reproducibility are unique to the human nervous system. Cerebral organoids and minimal lot-to-lot variability. have important applications in studying human brain development SIMPLE. Convenient format and easy-to-use protocol. and neurological disorders such as autism, schizophrenia or brain defects caused by Zika virus infection. The STEMdiff™ Cerebral Organoid Kit is a serum-free culture system that is designed to generate cerebral organoids from human embryonic stem (ES) cells observed during in vivo human brain development. -
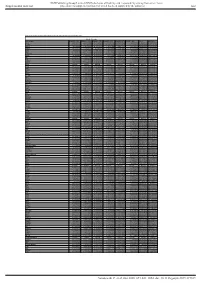
Supl Table 1 for Pdf.Xlsx
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Table S1. Proteomic analysis of liver tissues from 4-months old control and LPTENKO mice. Soluble Fraction Gene names LOG2FC CTL1 LOG2FC CTL2 LOG2FC CTL3 LOG2FC KO1 LOG2FC KO2 LOG2FC KO3 t-test adj Pvalue Aass 0.081686519 -0.097912098 0.016225579 -1.135271836 -0.860535624 -1.263037541 0.0011157 1.206072068 Acsm5 -0.125220746 0.071090866 0.054129881 -0.780692107 -0.882155692 -1.158378647 0.00189031 1.021713016 Gstm2 -0.12707966 0.572554941 -0.445475281 1.952813994 1.856518122 1.561376671 0.00517664 1.865316016 Gstm1 -0.029147714 0.298593425 -0.26944571 0.983159098 0.872028945 0.786125509 0.00721356 1.949464926 Fasn 0.08403202 -0.214149498 0.130117478 1.052480559 0.779734519 1.229308218 0.00383637 0.829422353 Upb1 -0.112438784 -0.137014769 0.249453553 -1.297732445 -1.181999331 -1.495303666 0.00102373 0.184442034 Selenbp2 0.266508271 0.084791964 -0.351300235 -2.040647809 -2.608780781 -2.039865739 0.00107121 0.165425127 Thrsp -0.15001075 0.177999342 -0.027988592 1.54283307 1.603048661 0.927443822 0.00453549 0.612858263 Hyi 0.142635733 -0.183013091 0.040377359 1.325929636 1.119934412 1.181313897 0.00044587 0.053553518 Eci1 -0.119041811 -0.014846366 0.133888177 -0.599970385 -0.555547972 -1.191875541 0.02292305 2.477981171 Aldh1a7 -0.095682449 -0.017781922 0.113464372 0.653424862 0.931724091 1.110750381 0.00356922 0.350756574 Pebp1 -0.06292058 -

Optimization of Cerebral Organoid Culture from Embr
CALIFORNIA STATE UNIVERSITY, NORTHRIDGE Growing Brains to Study Development: Optimization of Cerebral Organoid Culture from Embryonic Stem Cells A thesis submitted in partial fulfillment of the requirements For the degree of Master of Science in Biology By Jessie Erin Buth December 2015 The thesis of Jessie Erin Buth is approved: __________________________________ ____________________________________ Dr. Aida Metzenberg Date __________________________________ ____________________________________ Dr. Randy Cohen Date __________________________________ ____________________________________ Dr. Cindy Malone, Chair Date California State University, Northridge ii Table of Contents Signature Page ii List of Figures iii Abstract vi Chapter 1: Introduction 1 Chapter 2: Methods 15 Chapter 3: Results 23 Chapter 4: Discussion 50 References 55 Appendix: Supplemental Methods 59 List of Figures Figure 1: Human organoid protocol (Cell line H9) and morphology at various time points. 23 Figure 2: Review of cortex development. 24 Figure 3: V-bottomed 96-well plates improve aggregate formation and generates a thick neuroepithelial layer on day 18 compared to U-bottomed 96-well plates. 25 Figure 4: Plating at 9,000 cells per well on day 0 generates thickest continuous neuroepithelial layer compared to other cell numbers. 26 Figure 5: Addition of the BMP inhibitor (LDN193189) does not improve the efficiency of producing FOXG1+ cortical progenitors and inhibits formation of continuous N-cadherin+ apical membrane. 27 Figure 6: Fold change in gene expression of cortical markers increases as stem cell markers decrease in human cortical organoids over time by qPCR. 28 Figure 7: Human embryonic stem cells efficiently form cortical progenitors with 81.7% of total live cells per organoid positive for cortical marker FOXG1 at day 18. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Modelling Heme-Mediated Brain Injury Associated with Cerebral Malaria In
www.nature.com/scientificreports OPEN Modelling heme-mediated brain injury associated with cerebral malaria in human brain cortical organoids Adriana Harbuzariu1*, Sidney Pitts1, Juan Carlos Cespedes1, Keri Oxendine Harp1, Annette Nti1, Andrew P. Shaw2, Mingli Liu1 & Jonathan K. Stiles1* Human cerebral malaria (HCM), a severe encephalopathy associated with Plasmodium falciparum infection, has a 20–30% mortality rate and predominantly afects African children. The mechanisms mediating HCM-associated brain injury are difcult to study in human subjects, highlighting the urgent need for non-invasive ex vivo human models. HCM elevates the systemic levels of free heme, which damages the blood-brain barrier and neurons in distinct regions of the brain. We determined the efects of heme on induced pluripotent stem cells (iPSCs) and a three-dimensional cortical organoid system and assessed apoptosis and diferentiation. We evaluated biomarkers associated with heme-induced brain injury, including a pro-infammatory chemokine, CXCL-10, and its receptor, CXCR3, brain-derived neurotrophic factor (BDNF) and a receptor tyrosine-protein kinase, ERBB4, in the organoids. We then tested the neuroprotective efect of neuregulin-1 (NRG-1) against heme treatment in organoids. Neural stem and mature cells diferentially expressed CXCL-10, CXCR3, BDNF and ERBB4 in the developing organoids and in response to heme-induced neuronal injury. The organoids underwent apoptosis and structural changes that were attenuated by NRG-1. Thus, cortical organoids can be used to model heme-induced cortical brain injury associated with HCM pathogenesis as well as for testing agents that reduce brain injury and neurological sequelae. Brain organoids are self-assembled three-dimensional (3D) aggregates derived from pluripotent stem cells (iPSCs) with cell types and formations that mimic the embryonic human brain1–7. -

Stem Cells, Genome Editing and the Path to Translational Medicine
Stem Cells, Genome Editing, and the Path to Translational Medicine The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Soldner, Frank and Rudolf Jaenisch. "Stem Cells, Genome Editing, and the Path to Translational Medicine." Cell 175, 3 (October 2018): P615-632 © 2018 Elsevier Inc As Published http://dx.doi.org/10.1016/j.cell.2018.09.010 Publisher Elsevier BV Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/125973 Terms of Use Creative Commons Attribution-NonCommercial-NoDerivs License Detailed Terms http://creativecommons.org/licenses/by-nc-nd/4.0/ HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Cell. Author Manuscript Author manuscript; Manuscript Author available in PMC 2019 October 18. Published in final edited form as: Cell. 2018 October 18; 175(3): 615–632. doi:10.1016/j.cell.2018.09.010. Stem cells, genome editing and the path to translational medicine Frank Soldner1 and Rudolf Jaenisch1,2,3 1The Whitehead Institute, 455 Main Street, Cambridge, MA 02142, USA 2Department of Biology, Massachusetts Institute of Technology, 31 Ames Street, Cambridge, MA 02139, USA Summary The derivation of human embryonic stem cells (hESCs) and the stunning discovery that somatic cells can be reprogrammed into human induced pluripotent stem cells (hiPSCs) holds the promise to revolutionize biomedical research and regenerative medicine. In this review, we focus on disorders of the central nervous system and explore how advances in human pluripotent stem cells (hPSCs) coincide with evolutions in genome engineering and genomic technologies to provide realistic opportunities to tackle some of the most devastating complex disorders. -

Building Three-Dimensional Human Brain Organoids Sergiu P
Building three-dimensional human brain organoids Sergiu P. Paşca The organogenesis of the human central nervous system is an intricately culture methods, to self-organize into brain spheroids or organoids. orchestrated series of events that occurs over several months and These organoid cultures can be derived from any individual, can be ultimately gives rise to the circuits underlying cognition and behavior. guided to resemble specific brain regions, and can be employed to model There is a pressing need for developing reliable, realistic, and complex cell-cell interactions in assembloids and to build human circuits. personalized in vitro models of the human brain to advance our This emerging technology, in combination with bioengineering and other understanding of neural development, evolution, and disease. Pluripotent state-of-the-art methods for probing and manipulating neural tissue, has stem cells have the remarkable ability to dierentiate in vitro into any of the potential to bring insights into human brain organogenesis and the the germ layers and, with the advent of three-dimensional (3D) cell pathogenesis of neurological and psychiatric disorders. Brain organogenesis in vitro and in vivo Brain organogenesis in vitro and in vivo Brain assembloids Methods for generating neural cells in vitro aim to recapitulate Spinal Forebrain Forebrain Blastocyst Cerebral Dorsal Pallial-subpallial assembloid key stages of in vivo brain organogenesis. Folding of the Neural Neural cord Paired recordingOptogenetics Pharmacology fold crest cortex Glutamate uncaging ectoderm-derived neural plate gives rise to the neural tube, Embryonic Yo lk sac Region 1 Region 2Region 3 which becomes enlarged on the anterior side to form the stem cell Neural groove Ventral Inner cell forebrain in the central nervous system (CNS). -

Organoid Based Personalized Medicine: from Bench to Bedside Yaqi Li1,2†, Peiyuan Tang3†, Sanjun Cai1,2, Junjie Peng1,2 and Guoqiang Hua3,4*
Li et al. Cell Regeneration (2020) 9:21 https://doi.org/10.1186/s13619-020-00059-z REVIEW Open Access Organoid based personalized medicine: from bench to bedside Yaqi Li1,2†, Peiyuan Tang3†, Sanjun Cai1,2, Junjie Peng1,2 and Guoqiang Hua3,4* Abstract Three-dimensional cultured organoids have become a powerful in vitro research tool that preserves genetic, phenotypic and behavioral trait of in vivo organs, which can be established from both pluripotent stem cells and adult stem cells. Organoids derived from adult stem cells can be established directly from diseased epithelium and matched normal tissues, and organoids can also be genetically manipulated by CRISPR-Cas9 technology. Applications of organoids in basic research involve the modeling of human development and diseases, including genetic, infectious and malignant diseases. Importantly, accumulating evidence suggests that biobanks of patient- derived organoids for many cancers and cystic fibrosis have great value for drug development and personalized medicine. In addition, organoids hold promise for regenerative medicine. In the present review, we discuss the applications of organoids in the basic and translational research. Keywords: Organoids, Stem cells, Disease modeling, Biobanks, Personalized medicine Background phenocopy tumor heterogeneity is highly needed for re- Two-dimensional (2D) cultured cell lines have been the search on the mechanisms of cancer progression and ac- main in vitro research tool for the past decades. Cell quired drug resistance. In 1953, the first patient-derived lines are relatively cheap, easy to handle and can be ap- xenograft (PDX) models were successfully established plied to multiple experimental techniques. However, the (Toolan 1953). In this model, primary tumor tissue is establishment of a cell line is time-consuming and transplanted into immune-deficient mice, while tumor involves extensive genetic and phenotypic adaption to structure and the relative proportion of tumor cells and culture conditions. -

Amsterdamprogram2019 022019.Pdf
3D Cell Culture and Analysis Explore new dimensions in 3D cell modeling As we look to the future of innovation, creating more physiologically relevant models through the study of organoids and spheroids is becoming increasingly important. These three-dimensional (3D) models not only mimic cell functions, they also allow for more robust testing of compounds and a more representative analysis. That’s why we’ve compiled a collection of tested products, protocols, and seminal publications to help you accelerate the development of more realistic cellular models. Find out more about culturing and analyzing organoids and spheroids at thermofi sher.com/organoid For Research Use Only. Not for use in diagnostic procedures. © 2018 Thermo Fisher Scientifi c Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientifi c and its subsidiaries unless otherwise specifi ed. COL23163 1218 PG1965-PJT4432-COL23163-3D-CellModeling-SSCR-PrintAd-Global.indd 1 12/20/18 2:25 PM Stem Cells & Organoids in Development & Disease AMSTERDAM NETHERLANDS Welcome Dear Colleagues, On behalf of the International Society for Stem Cell Research (ISSCR), we warmly welcome you to beautiful Amsterdam for the International Symposia, “Stem Cells & Organoids in Development & Disease.” A leader in life science and health technologies, Amsterdam was recently ranked as one of the top academic and biomedical communities in Europe. Amsterdam’s culture of education and innovation makes it the perfect setting for this timely and important meeting. Research using organoids is rapidly advancing the field of stem cell science. Recent work has demonstrated that stem cells retain self-organizing properties in culture, recapitulating in vivo processes and forming organ- like tissues. -

Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces Cerevisiae
life Review Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces cerevisiae Leticia V. R. Franco 1,2,* , Luca Bremner 1 and Mario H. Barros 2 1 Department of Biological Sciences, Columbia University, New York, NY 10027, USA; [email protected] 2 Department of Microbiology,Institute of Biomedical Sciences, Universidade de Sao Paulo, Sao Paulo 05508-900, Brazil; [email protected] * Correspondence: [email protected] Received: 27 October 2020; Accepted: 19 November 2020; Published: 23 November 2020 Abstract: The ease with which the unicellular yeast Saccharomyces cerevisiae can be manipulated genetically and biochemically has established this organism as a good model for the study of human mitochondrial diseases. The combined use of biochemical and molecular genetic tools has been instrumental in elucidating the functions of numerous yeast nuclear gene products with human homologs that affect a large number of metabolic and biological processes, including those housed in mitochondria. These include structural and catalytic subunits of enzymes and protein factors that impinge on the biogenesis of the respiratory chain. This article will review what is currently known about the genetics and clinical phenotypes of mitochondrial diseases of the respiratory chain and ATP synthase, with special emphasis on the contribution of information gained from pet mutants with mutations in nuclear genes that impair mitochondrial respiration. Our intent is to provide the yeast mitochondrial specialist with basic knowledge of human mitochondrial pathologies and the human specialist with information on how genes that directly and indirectly affect respiration were identified and characterized in yeast. Keywords: mitochondrial diseases; respiratory chain; yeast; Saccharomyces cerevisiae; pet mutants 1. -

Improving Tissue Differentiation in Cerebral Organoids
Improving tissue differentiation in cerebral organoids Technical Journal Club Manfredi Carta 16.06.2020 Contents Introduction Protocol overview 1. hPSCs are incubated in human ES medium with low bFGF4 and ROCK inhibitor embryoid bodies (= 3D aggregates of hPSCs) 2. Transfer to neural induction medium (DMEM/F12, N2, GluMax, amino acids, heparin) 3. Transfer to Matrigel, grow in differentiation media (DMEM/F12, Neurobasal, B27 w/o Vitamin A, N2, β-ME, insulin, GluMax, amino acids) 4. Add Vitamin A to media, grow in spinning bioreactor bFGF4: basic fibrobl. growth factor 4 ROCK: Rho-associated protein kinase Spinning conditions Tissues grown in spinning bioreactor • developed contained large fluid-filled cavities (similar to ventricles) • apical localisation of neural N-cadherin • Were larger and more continuous than tissues grown in stationary suspension Fluid-filled cavity Retina-like pigmented epithelium SOX2+: neural progenitors Scale bars: 200µm TUJ1+: neurons Cerebral organoids: Anatomy Forebrain Choroid plexus Molecular layer Meninges Choroid plexus • Spinning conditions: anatomy reminiscent of brain regions • Organoids lacked vasculature, limited oxygen and nutrient diffusion: cell death in internal areas (TUNEL stain) Mammalian brain development • Neuroepithelium expands to generate radial glial stem cells (RG) • RG divide at the apical surface within the ventricular zone (VZ), generate neurons and intermediate progenitor cells (IPCs) • IPCs populate the subventricular zone (SVZ), neurons migrate through intermediate zone (IZ) to -

Midbrain Organoids with an SNCA Gene Triplication Model Key Features of Synucleinopathy
bioRxiv preprint doi: https://doi.org/10.1101/2021.04.12.439480; this version posted April 12, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Midbrain organoids with an SNCA gene triplication model key features of synucleinopathy Running Title: Midbrain organoids model synucleinopathy Nguyen-Vi Mohamed1, Julien Sirois1, Janani Ramamurthy1, Meghna Mathur1, Paula Lépine1, Eric Deneault1, Gilles Maussion1, Michael Nicouleau1, Carol X.-Q. Chen1, Narges Abdian1, Vincent Soubannier1, Eddie Cai1, Harris Nami1, Rhalena A. Thomas1, Mahdieh Tabatabaei1,2, Lenore K. Beitel1, Karamjit Singh Dolt3, Jason Karamchandani1,2, Tilo Kunath3, Edward A. Fon1, Thomas M. Durcan1 1 Early Drug Discovery Unit (EDDU), Montreal Neurological Institute-Hospital, Department of Neurology and Neurosurgery, McGill University, 3801 University Street, Montreal, Quebec, H3A 2B4, Canada 2 C-BIG Biorepository (C-BIG), Montreal Neurological Institute-Hospital, McGill University, 3801 University Street, Montreal, Quebec, H3A 2B4, Canada 3 Centre for Regenerative Medicine, School of Biological Sciences, University of Edinburgh, 5 Little France Drive, Edinburgh EH16 4UU, UK Corresponding authors: Thomas M. Durcan: [email protected] and Edward A. Fon: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2021.04.12.439480; this version posted April 12, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license.