Molecular and Morphological Support for a Florida Origin of the Cuban
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nature Policy Plan
Ministry of Public Housing, Spatial Planning, Environment And Infrastructure Ministerie van Volkshuisvesting, Ruimtelijke Ordening, Milieu en Infrastructuur Nature Policy Plan 2021 – 2025 Established/Approved: Date: By: i Nature Policy Plan Sint Maarten 2021 – 2025 “We the people of Sint Maarten: RESOLVED to provide for the continuing preservation of nature and the environment”. Constitution of Sint Maarten ii Nature Policy Plan Sint Maarten 2021 – 2025 Nature Policy Plan Sint Maarten 2021 – 2025 Ministry of Public Housing, Spatial Planning, Environment and Infrastructure (Ministry of VROMI) Address: Government of Sint Maarten Ministry of VROMI Soualiga Road #1 Pond Island, Great Bay Sint Maarten Contact: [email protected] [email protected] iii Nature Policy Plan Sint Maarten 2021 – 2025 Lignum Vitae (Guaiacum officinale) iv Photo by: Mark Yokoyama Nature Policy Plan Sint Maarten 2021 – 2025 Acknowledgments In writing the Nature Policy Plan Sint Maarten 2021 – 2025, the Ministry of VROMI consulted several government ministries, and external stakeholders including private sector entities and NGO’s. Some were engaged in the preparation of the policy from the onset; others were part of a review of the policy and stakeholder meetings. The Ministry of VROMI acknowledges and appreciates the time and effort of the stakeholders who contributed to the formulation of this Nature Policy Plan, which provides insights into the current state of affairs of nature on Sint Maarten and the proposed way forward on nature conservation -

Comparative Anatomy of the Fig Wall (Ficus, Moraceae)
Botany Comparative anatomy of the fig wall (Ficus, Moraceae) Journal: Botany Manuscript ID cjb-2018-0192.R2 Manuscript Type: Article Date Submitted by the 12-Mar-2019 Author: Complete List of Authors: Fan, Kang-Yu; National Taiwan University, Institute of Ecology and Evolutionary Biology Bain, Anthony; national Sun yat-sen university, Department of biological sciences; National Taiwan University, Institute of Ecology and Evolutionary Biology Tzeng, Hsy-Yu; National Chung Hsing University, Department of Forestry Chiang, Yun-Peng;Draft National Taiwan University, Institute of Ecology and Evolutionary Biology Chou, Lien-Siang; National Taiwan University, Institute of Ecology and Evolutionary Biology Kuo-Huang, Ling-Long; National Taiwan University, Institute of Ecology and Evolutionary Biology Keyword: Comparative Anatomy, Ficus, Histology, Inflorescence Is the invited manuscript for consideration in a Special Not applicable (regular submission) Issue? : https://mc06.manuscriptcentral.com/botany-pubs Page 1 of 29 Botany Comparative anatomy of the fig wall (Ficus, Moraceae) Kang-Yu Fana, Anthony Baina,b *, Hsy-Yu Tzengc, Yun-Peng Chianga, Lien-Siang Choua, Ling-Long Kuo-Huanga a Institute of Ecology and Evolutionary Biology, College of Life Sciences, National Taiwan University, 1, Sec. 4, Roosevelt Road, Taipei, 10617, Taiwan b current address: Department of Biological Sciences, National Sun Yat-sen University, 70 Lien-Hai road, Kaohsiung, Taiwan.Draft c Department of Forestry, National Chung Hsing University, 145 Xingda Rd., South Dist., Taichung, 402, Taiwan. * Corresponding author: [email protected]; Tel: +886-75252000-3617; Fax: +886-75253609. 1 https://mc06.manuscriptcentral.com/botany-pubs Botany Page 2 of 29 Abstract The genus Ficus is unique by its closed inflorescence (fig) holding all flowers inside its cavity, which is isolated from the outside world by a fleshy barrier: the fig wall. -

Oaks (Quercus Spp.): a Brief History
Publication WSFNR-20-25A April 2020 Oaks (Quercus spp.): A Brief History Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources Quercus (oak) is the largest tree genus in temperate and sub-tropical areas of the Northern Hemisphere with an extensive distribution. (Denk et.al. 2010) Oaks are the most dominant trees of North America both in species number and biomass. (Hipp et.al. 2018) The three North America oak groups (white, red / black, and golden-cup) represent roughly 60% (~255) of the ~435 species within the Quercus genus worldwide. (Hipp et.al. 2018; McVay et.al. 2017a) Oak group development over time helped determine current species, and can suggest relationships which foster hybridization. The red / black and white oaks developed during a warm phase in global climate at high latitudes in what today is the boreal forest zone. From this northern location, both oak groups spread together southward across the continent splitting into a large eastern United States pathway, and much smaller western and far western paths. Both species groups spread into the eastern United States, then southward, and continued into Mexico and Central America as far as Columbia. (Hipp et.al. 2018) Today, Mexico is considered the world center of oak diversity. (Hipp et.al. 2018) Figure 1 shows genus, sub-genus and sections of Quercus (oak). History of Oak Species Groups Oaks developed under much different climates and environments than today. By examining how oaks developed and diversified into small, closely related groups, the native set of Georgia oak species can be better appreciated and understood in how they are related, share gene sets, or hybridize. -

Origen I Evolució Dels Endemismes Vegetals De Les Illes Del Carib
Òscar Castillo Agudo Final Degree Project Origin and evolution of the Biology (2020) Caribbean Islands endemic plants The Islands Geological History The Caribbean Islands, also known Greater Antilles: originated as a submerged volcanic arc (Proto-Greater as the West Indies, are conformed Antilles) between North and South America (130 Ma.) The last time the by three archipelago. These islands islands emerged as a landmass was 49 Ma and later Cuba, the have been categorized as one of Hispaniola and Puerto Rico split (25-20 Ma). the world’s biodiversity hotspots Lesser Antilles: originated in two times as oceanic islands. with conservation priority as the Bahames: got the current configuration in the Eocene but the land ecosystem is threatened by loss of surface and connections changed drastically due to the fluctuations in habitat from anthropogenic origin. the sea level. Figure 1. Source 8. GAARlandia: an hypothetical land bridge connecting the Biodiversity: some numbers north of South America and the Greater Antilles for 3 million 12.847 taxa of seed plants, 10.948 years at Eocene-Oligocene are native and 7.868 are endemic transition (c. 33 Ma). (72% out of the native). 1.447 native genera with 181 endemic and 10 nearly endemic, Biogeography Figure 3. Source 9. 47,5% of the endemic are monotypic. 2 ways of colonization of the islands from the continent: The Greater Antilles hosts most of Vicariance: the endemic genera. First interpretations considered the Proto-Antilles to be connected with Figure 2. Source 1. the continent but now we know they were submerged. Now it is Isolation atribuited to GAARAlandia acting as a low-land connection for the biota. -

27 Skunk Vine
27 SKUNK VINE R. W. Pemberton and P. D. Pratt U.S. Department of Agriculture, Agricultural Research Service, Invasive Plant Research Laboratory, Fort Lauderdale, Florida, USA tion of livestock, however, are unknown (Gann and PEST STATUS OF WEED Gordon, 1998). In urban landscapes, this vine en- Skunk vine, Paederia foetida L. (Fig. 1), is a recently twines branches of woody ornamental plants and also recognized weedy vine of natural areas in Florida that spreads horizontally through lawns, rooting at the is spreading into other parts of the southern United nodes (Martin, 1995). In westcentral Florida, P. States. The weed, which is native to Asia, appears to foetida is considered the most troublesome weed have the potential to spread well beyond the South along roadside right-of-ways (W. Moriaty, pers. to the northeastern states. Control of the plant by comm.), and it also entangles power lines and associ- chemical or mechanical means damages valued veg- ated structures (Martin, 1995). etation supporting the vine. Skunk vine is a Category On the island of Hawaii, P. foetida is a very se- I Florida Exotic Pest Plant Council weed (Langeland rious weed in nurseries producing ornamental foli- and Craddock Burks, 1998), a listing that groups the age plants (Pemberton, pers. obs.). The weed infests plant with the most invasive weed species in Florida. field plantings used for propagation. Control of the weed is very difficult because stock plants are easily injured if herbicides are applied. At times, growers have had to abandon or destroy stock plants that have become overgrown by skunk vine. -

Ernodea Littoralis Golden Creeper1 Edward F
FPS196 Ernodea littoralis Golden Creeper1 Edward F. Gilman2 Introduction Origin: native to Florida Uses: ground cover The golden creeper is a 1- to 3-foot-tall, prostrate ground Availability: somewhat available, may have to go out of the cover that is native to south Florida beaches (Fig. 1). This region to find the plant plant has small, light green, succulent leaves borne on bright red stems that help it to survive in dry conditions. Inconspicuous, pinkish white, tubular flowers occur throughout the year and are followed by attractive golden berries. These golden berries, in part, give this plant its common name. Figure 2. Shaded area represents potential planting range. Description Height: 1 to 3 feet Figure 1. Golden creeper. Spread: depends upon supporting structure Plant habit: spreading; prostrate (flat) General Information Plant density: moderate Scientific name: Ernodea littoralis Growth rate: moderate Pronunciation: air-NOE-dee-uh lit-taw-RAIL-liss Texture: fine Common name(s): golden creeper Family: Rubiaceae Foliage Plant type: ground cover Leaf arrangement: opposite/subopposite USDA hardiness zones: 10B through 11 (Fig. 2) Leaf type: simple Planting month for zone 10 and 11: year round Leaf margin: entire 1. This document is FPS196, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date October 1999. Reviewed February 2014. Visit the EDIS website at http://edis.ifas.ufl.edu. 2. Edward F. Gilman, professor, Environmental Horticulture Department, UF/IFAS Extension, Gainesville, FL 32611. The Institute of Food and Agricultural Sciences (IFAS) is an Equal Opportunity Institution authorized to provide research, educational information and other services only to individuals and institutions that function with non-discrimination with respect to race, creed, color, religion, age, disability, sex, sexual orientation, marital status, national origin, political opinions or affiliations. -

Appendix A. Plant Species Known to Occur at Canaveral National Seashore
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Vegetation Community Monitoring at Canaveral National Seashore, 2009 Natural Resource Data Series NPS/SECN/NRDS—2012/256 ON THE COVER Pitted stripeseed (Piriqueta cistoides ssp. caroliniana) Photograph by Sarah L. Corbett. Vegetation Community Monitoring at Canaveral National Seashore, 2009 Natural Resource Report NPS/SECN/NRDS—2012/256 Michael W. Byrne and Sarah L. Corbett USDI National Park Service Southeast Coast Inventory and Monitoring Network Cumberland Island National Seashore 101 Wheeler Street Saint Marys, Georgia, 31558 and Joseph C. DeVivo USDI National Park Service Southeast Coast Inventory and Monitoring Network University of Georgia 160 Phoenix Road, Phillips Lab Athens, Georgia, 30605 March 2012 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Data Series is intended for the timely release of basic data sets and data summaries. Care has been taken to assure accuracy of raw data values, but a thorough analysis and interpretation of the data has not been completed. Consequently, the initial analyses of data in this report are provisional and subject to change. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -
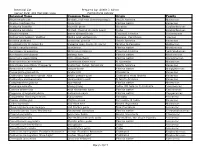
Botanical Name Common Name Origin Family
Botanical List Prepared by: Arielle J. Simon Corner Park: 401 Hampton Lane Horticultural Advisor Botanical Name Common Name Origin Family Abutilon pictum fireball, red vein flowering maple South America Malvaceae Acacia choriophylla cinnecord Florida native Fabaceae Acalypha hispida chenille plant Oceania Euphorbiaceae Acalypha pendula firetail, trailing chenille plant Cuba Euphorbiaceae Aloysia virgata sweet almond bush Tropical America Verbenaceae Anthurium hookeri 'Ruffles' bird's nest anthurium Guyana, Caribbean Araceae Arachis glabrata perennial peanut South America Fabaceae Arachnothryx leucophylla Panama rose, huele de noche Mexico to Panama Rubiaceae Ardisia escallonioides marlberry Florida native Myrsinaceae Asclepias curassavica Mexican milkweed Tropical America Asclepiadaceae Blechnum serrulatum swamp fern Florida native Blechnaceae Bourreria cassinifolia little strongback Florida native Boraginaceae Brachychiton acerifolius Australian flame tree N Australia Malvaceae Brunfelsia pauciflora 'Compacta' yesterday, today, tomorrow South America Fabaceae Byrsonima lucida locust-berry Florida native Malpighiaceae Caesalpinia granadillo bridal veil Venezuela Fabaceae Calliandra haematocephala 'Alba' white powder-puff Cultivated from Bolivia Fabaceae Calliandra surinamensis pink powder-puff N South America Fabaceae Calyptranthes pallens spicewood Florida native Myrtaceae Cananga odorata ylang-ylang India, SE Asia to N Australia Annonaceae Canella winterana wild cinnamon bark Florida native Canellaceae Capparis cynophallophora Jamaican -

UFFLORIDA IFAS Extension
ENH854 UFFLORIDA IFAS Extension Low-Maintenance Landscape Plants for South Florida1 Jody Haynes, John McLaughlin, Laura Vasquez, Adrian Hunsberger2 Introduction The term "low-maintenance" refers to a plant that does not require frequent maintenance-such as This publication was developed in response to regular watering, pruning, or spraying-to remain requests from participants in the Florida Yards & healthy and to maintain an acceptable aesthetic Neighborhoods (FYN) program in Miami-Dade quality. A low-maintenance plant has low fertilizer County for a list of recommended landscape plants requirements and few pest and disease problems. In suitable for south Florida. The resulting list includes addition, low-maintenance plants suitable for south over 350 low-maintenance plants. The following Florida must also be adapted to--or at least information is included for each species: common tolerate-our poor, alkaline, sand- or limestone-based name, scientific name, maximum size, growth rate soils. (vines only), light preference, salt tolerance, and other useful characteristics. An additional criterion for the plants on this list was that they are not listed as being invasive by the Criteria Florida Exotic Pest Plant Council (FLEPPC, 2001), or restricted by any federal, state, or local laws This section will describe the criteria by which (Burks, 2000). Miami-Dade County does have plants were selected. It is important to note, first, that restrictions for planting certain species within 500 even the most drought-tolerant plants require feet of native habitats they are known to invade watering during the establishment period. Although (Miami-Dade County, 2001); caution statements are this period varies among species and site conditions, provided for these species. -

Muller Files MS.01
http://oac.cdlib.org/findaid/ark:/13030/c8xg9z0q No online items Guide to the Cornelius H. Muller Files MS.01 Finding aid prepared by Laurie Hannah. Arrangement and description of this collection funded by a grant from the Institute of Museum and Library Services. Cheadle Center for Biodiversity and Ecological Restoration. C.H. Muller Library University of California Harder South MS 9615 Santa Barbara, CA, 93106 805-893-2401 Guide to the Cornelius H. Muller MS.01 1 Files MS.01 Title: Muller (Cornelius H.) Files Identifier/Call Number: MS.01 Contributing Institution: Cheadle Center for Biodiversity and Ecological Restoration. C.H. Muller Library Language of Material: English Physical Description: 30.75 Linear feet: 8 record cartons, 4 photo boxes, 12 oversize folders, artifacts Date (inclusive): 1931-1996 Language of Material: Some publications in German and some correspondence in Spanish, French, and German. Abstract: Correspondence, publications, field notes, research notes, and photographs relating to the career of plant ecologist C.H. Muller. creator: Muller, Cornelius H. Arrangement note The papers are organized into six series as follows. Series 1. Correspondence, 1936-1996. Arrangement is alphabetical by correspondent and then chronological within each folder. Subject files are arranged alphabetically by correspondent. Series 2. Research and Publications; Series 3. Faculty and Professional Papers; Series 4. Personal Papers; Series 5. Maps; Series 6 Artifacts. Conditions Governing Access note Collection open for research. Immediate Source of Acquisition note Donated in two gifts in 2005 and 2007 by Robert Muller. Preferred Citation note Cornelius H. Muller Files. MS-01. Cheadle Center for Biodiversity and Ecological Restoration, University of California, Santa Barbara. -

PARTV the VEGETATION MAP of CUBA Paklv the Vegetation Map of Cuba 22 the Main Vegetation Types of Cuba
PARTV THE VEGETATION MAP OF CUBA PAKlV The vegetation map of Cuba 22 The main vegetation types of Cuba . 389 22.1 Rainforests . 389 22.1.1 Submontane rainforests (Calophyllo- Carapetum guianensis) . 389 22.1.2 Wetmontanerainforests (Ocoteo-Magnolietalia) ..................... 392 22.1.3 Semi-arid montane serpentine rainforests (Podocarpo-Sloanetalia) .. , 396 • 22.1.4 Cloudforests or mossy forests (Weinmannio-Cyrilletalia) . 398 22.1.5 Semi-arid montane serpentine shrubwoods (Clusio-llicetalia) 400 22.1.6 Elfin thickets (Jlici-Myricion cacuminis) ............................... 402 22.2 Seasonal evergreen forests or seasonal rainforests . 404 22.2.1 Lowland seasonal rainforests . 404 22.2.2 Submontane seasonalrainforests (Oxandro-Dipholietum) ............ 405 22.3 Semi-deciduous forests . 410 ~ 22.3.1 Semi-deciduous mesophytic forests (Oxandro-Burseretalia) . 410 22.3.2 Semi-deciduous xerophytic forests . .. .. 415 22.4 Tropical karstic forests . 416 22.4.1 Species rich karstic forests of western Cuba (Spathelio-Gaussion) 417 ,..~ 22.4.2 Species poor karstic forests of western Cuba (Thrinacion morrisii) . 418 22.4.3 Karstic forests of eastern Cuba (Tabebuio-Coccothrinacion) 418 22.4.4 Montane karstic forests (Tabebuio-Garryetum) .......... : . 419 22.5 Dry forests and shrubwoods . .. 419 22.5.1 Dry evergreen forests (Eugenio-Metopietalia toxiferi) ................. 420 22.5.2 Dry, thorny limestone shrubwoods ( Lantano-Cordietalia) . 423 22.5.3 Dry lowland serpentine shrubwoods (Phyllantho-Neobracetalia) ....... ·425 22.5.4 Semi-dry lowland serpentine shrublands (Ariadno-Phyllanthetalia) ..... 426 22.6 Semi-desert cactus scrubs (Consoleo-Ritterocereion hystricis) ................... 427 22. 7 Coniferous forests . 431 22. 7 .1 Pinus tropicalis forests on sand (Acoelorrapho- Pinion tropicalis) 431 22.7.2 Pinus caribaea and mixed oak-pine forests on slatey rocks (Pachyantho- Pinion caribaeae) .............................................................. -

(Rubiaceae), a Uniquely Distylous, Cleistogamous Species Eric (Eric Hunter) Jones
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2012 Floral Morphology and Development in Houstonia Procumbens (Rubiaceae), a Uniquely Distylous, Cleistogamous Species Eric (Eric Hunter) Jones Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES FLORAL MORPHOLOGY AND DEVELOPMENT IN HOUSTONIA PROCUMBENS (RUBIACEAE), A UNIQUELY DISTYLOUS, CLEISTOGAMOUS SPECIES By ERIC JONES A dissertation submitted to the Department of Biological Science in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Summer Semester, 2012 Eric Jones defended this dissertation on June 11, 2012. The members of the supervisory committee were: Austin Mast Professor Directing Dissertation Matthew Day University Representative Hank W. Bass Committee Member Wu-Min Deng Committee Member Alice A. Winn Committee Member The Graduate School has verified and approved the above-named committee members, and certifies that the dissertation has been approved in accordance with university requirements. ii I hereby dedicate this work and the effort it represents to my parents Leroy E. Jones and Helen M. Jones for their love and support throughout my entire life. I have had the pleasure of working with my father as a collaborator on this project and his support and help have been invaluable in that regard. Unfortunately my mother did not live to see me accomplish this goal and I can only hope that somehow she knows how grateful I am for all she’s done. iii ACKNOWLEDGEMENTS I would like to acknowledge the members of my committee for their guidance and support, in particular Austin Mast for his patience and dedication to my success in this endeavor, Hank W.