Live Oak Pub 10-23
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Anatomy of the Fig Wall (Ficus, Moraceae)
Botany Comparative anatomy of the fig wall (Ficus, Moraceae) Journal: Botany Manuscript ID cjb-2018-0192.R2 Manuscript Type: Article Date Submitted by the 12-Mar-2019 Author: Complete List of Authors: Fan, Kang-Yu; National Taiwan University, Institute of Ecology and Evolutionary Biology Bain, Anthony; national Sun yat-sen university, Department of biological sciences; National Taiwan University, Institute of Ecology and Evolutionary Biology Tzeng, Hsy-Yu; National Chung Hsing University, Department of Forestry Chiang, Yun-Peng;Draft National Taiwan University, Institute of Ecology and Evolutionary Biology Chou, Lien-Siang; National Taiwan University, Institute of Ecology and Evolutionary Biology Kuo-Huang, Ling-Long; National Taiwan University, Institute of Ecology and Evolutionary Biology Keyword: Comparative Anatomy, Ficus, Histology, Inflorescence Is the invited manuscript for consideration in a Special Not applicable (regular submission) Issue? : https://mc06.manuscriptcentral.com/botany-pubs Page 1 of 29 Botany Comparative anatomy of the fig wall (Ficus, Moraceae) Kang-Yu Fana, Anthony Baina,b *, Hsy-Yu Tzengc, Yun-Peng Chianga, Lien-Siang Choua, Ling-Long Kuo-Huanga a Institute of Ecology and Evolutionary Biology, College of Life Sciences, National Taiwan University, 1, Sec. 4, Roosevelt Road, Taipei, 10617, Taiwan b current address: Department of Biological Sciences, National Sun Yat-sen University, 70 Lien-Hai road, Kaohsiung, Taiwan.Draft c Department of Forestry, National Chung Hsing University, 145 Xingda Rd., South Dist., Taichung, 402, Taiwan. * Corresponding author: [email protected]; Tel: +886-75252000-3617; Fax: +886-75253609. 1 https://mc06.manuscriptcentral.com/botany-pubs Botany Page 2 of 29 Abstract The genus Ficus is unique by its closed inflorescence (fig) holding all flowers inside its cavity, which is isolated from the outside world by a fleshy barrier: the fig wall. -

Oaks (Quercus Spp.): a Brief History
Publication WSFNR-20-25A April 2020 Oaks (Quercus spp.): A Brief History Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources Quercus (oak) is the largest tree genus in temperate and sub-tropical areas of the Northern Hemisphere with an extensive distribution. (Denk et.al. 2010) Oaks are the most dominant trees of North America both in species number and biomass. (Hipp et.al. 2018) The three North America oak groups (white, red / black, and golden-cup) represent roughly 60% (~255) of the ~435 species within the Quercus genus worldwide. (Hipp et.al. 2018; McVay et.al. 2017a) Oak group development over time helped determine current species, and can suggest relationships which foster hybridization. The red / black and white oaks developed during a warm phase in global climate at high latitudes in what today is the boreal forest zone. From this northern location, both oak groups spread together southward across the continent splitting into a large eastern United States pathway, and much smaller western and far western paths. Both species groups spread into the eastern United States, then southward, and continued into Mexico and Central America as far as Columbia. (Hipp et.al. 2018) Today, Mexico is considered the world center of oak diversity. (Hipp et.al. 2018) Figure 1 shows genus, sub-genus and sections of Quercus (oak). History of Oak Species Groups Oaks developed under much different climates and environments than today. By examining how oaks developed and diversified into small, closely related groups, the native set of Georgia oak species can be better appreciated and understood in how they are related, share gene sets, or hybridize. -

ISB: Atlas of Florida Vascular Plants
Longleaf Pine Preserve Plant List Acanthaceae Asteraceae Wild Petunia Ruellia caroliniensis White Aster Aster sp. Saltbush Baccharis halimifolia Adoxaceae Begger-ticks Bidens mitis Walter's Viburnum Viburnum obovatum Deer Tongue Carphephorus paniculatus Pineland Daisy Chaptalia tomentosa Alismataceae Goldenaster Chrysopsis gossypina Duck Potato Sagittaria latifolia Cow Thistle Cirsium horridulum Tickseed Coreopsis leavenworthii Altingiaceae Elephant's foot Elephantopus elatus Sweetgum Liquidambar styraciflua Oakleaf Fleabane Erigeron foliosus var. foliosus Fleabane Erigeron sp. Amaryllidaceae Prairie Fleabane Erigeron strigosus Simpson's rain lily Zephyranthes simpsonii Fleabane Erigeron vernus Dog Fennel Eupatorium capillifolium Anacardiaceae Dog Fennel Eupatorium compositifolium Winged Sumac Rhus copallinum Dog Fennel Eupatorium spp. Poison Ivy Toxicodendron radicans Slender Flattop Goldenrod Euthamia caroliniana Flat-topped goldenrod Euthamia minor Annonaceae Cudweed Gamochaeta antillana Flag Pawpaw Asimina obovata Sneezeweed Helenium pinnatifidum Dwarf Pawpaw Asimina pygmea Blazing Star Liatris sp. Pawpaw Asimina reticulata Roserush Lygodesmia aphylla Rugel's pawpaw Deeringothamnus rugelii Hempweed Mikania cordifolia White Topped Aster Oclemena reticulata Apiaceae Goldenaster Pityopsis graminifolia Button Rattlesnake Master Eryngium yuccifolium Rosy Camphorweed Pluchea rosea Dollarweed Hydrocotyle sp. Pluchea Pluchea spp. Mock Bishopweed Ptilimnium capillaceum Rabbit Tobacco Pseudognaphalium obtusifolium Blackroot Pterocaulon virgatum -

Bromeliads Bromeliads Are a Family of Plants (Bromeliaceae, the Pineapple Family) Native to Tropical North and South America
A Horticulture Information article from the Wisconsin Master Gardener website, posted 19 March 2012 Bromeliads Bromeliads are a family of plants (Bromeliaceae, the pineapple family) native to tropical North and South America. Europeans fi rst found out about bromeliads on Columbus’ second trip to the New World in 1493, where the pineapple (Ananas sp.) was being cultivated by the Carib tribe in the West Indies. The commercial pineapple (Ananas comosus) is native to southern Brazil and Paraguay. After the colonization of the New World it was rapidly transported to all areas of the tropics, and now is widely grown in tropical and sub- tropical areas. The only A collection of bromeliads placed on a tree at Costa Flores, Costa Rica. bromeliad to occur north of the tropics is Spanish “moss” (Tillandsia usneoides). It is neither Spanish nor a moss, but an epiphytic bromeliad. It doesn’t look much like a typical Commercial pineapple, Ananas comosus, bromeliad, though, with its long scaly stems and reduced in the fi eld. fl owers. Bromeliads are monocots, many of which, like their grass relatives, have a special form of photosynthesis that uses a variation of the more usual biochemical pathways to allow them to use water more effi ciently. Even though they come from the tropics, this helps those that are epiphytes contend with life in the treetops where there is limited water and a real danger of drying out. There are about 2500 species Many bromeliads are tropical and several thousand hybrids epiphytes. and cultivars. Many have brightly colored leaves, fl owers or fruit, and range in size from moss-like species of Tillandsia to the enormous Puya raimondii from the Andes which produces a fl owering stem up to 15 feet tall. -
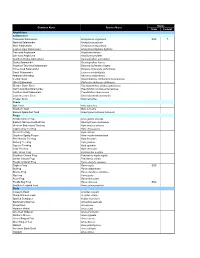
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Molecular and Morphological Support for a Florida Origin of the Cuban
Journal of Biogeography (J. Biogeogr.) (2011) SPECIAL Molecular and morphological support ISSUE for a Florida origin of the Cuban oak Paul F. Gugger* and Jeannine Cavender-Bares Department of Ecology, Evolution and ABSTRACT Behavior, University of Minnesota, 100 Ecology Aim The origins of the Cuban biota are of long-standing interest in Building, 1987 Upper Buford Circle, Saint Paul, MN, USA biogeography, and the source of a small live oak (Quercus series Virentes) population on Cuba remains unresolved. Based on morphological evidence, previous authors have hypothesized a Florida origin from either Q. geminata or Q. virginiana or both; a Mexican origin from Q. oleoides; or a hybrid origin from both sources. We use molecular data and taxonomically informative leaf morphology to identify the source species and timing of colonization. Location Cuba, Central America, Mexico and the south-eastern United States. Methods We collected representative samples of Cuban oaks and each putative source species and genotyped each sample at 12 nuclear microsatellites and two chloroplast DNA sequences. We estimated population structure using a Bayesian clustering analysis and F-statistics, pairwise migration rates among taxa, and divergence time using an isolation-with-migration model. We measured seven leaf traits and conducted an analysis of similarity (ANOSIM) to determine which putative source species was most similar to Cuban oaks. Results Cuban oak contains one chloroplast DNA haplotype, which is common in southern Florida. Bayesian clustering analysis of microsatellites revealed that the Cuban oak forms a distinct and pure population cluster, and F-statistics showed that Cuban oaks are differentiated least from Q. virginiana and most from Q. -

The Effect of Season of Fire on the Recovery of Florida Scrub
2B.7 THE EFFECT OF SEASON OF FIRE ON THE RECOVERY OF FLORIDA SCRUB Tammy E. Foster* and Paul A. Schmalzer Dynamac Corporation, Kennedy Space Center, Florida 1. ABSTRACT∗ scrub, rosemary scrub, and scrubby flatwoods (Myers 1990, Abrahamson and Hartnett 1990). Florida scrub is a xeromorphic shrubland that is Florida scrub is habitat for threatened and maintained by frequent fires. Historically, these fires endangered plants and animals (Christman and Judd occurred during the summer due to lightning ignition. 1990, Stout and Marion 1993, Stout 2001). Today, Florida scrub is often managed by the use of Management of remaining scrub is critical to the survival prescribed burning. Prescribed burning of scrub has of these species. Scrub is a fire-maintained system been implemented on Kennedy Space Center/Merritt (Myers 1990, Menges 1999), and recovery after fire of Island National Wildlife Refuge (KSC/MINWR) since oak scrub and scrubby flatwoods is primarily through 1981, with burns being carried out throughout the year. sprouting and, in some species, clonal spread of the The impacts of the season of burn on recovery are not dominant shrubs (Abrahamson 1984a, 1984b, known. Long-term monitoring of scrub regeneration has Schmalzer and Hinkle 1992a, Menges and Hawkes been conducted since the early-1980’s at KSC/MINWR 1998). Scrub naturally burned during the summer using permanent 15 m line-intercept transects. We months due to lightning ignition. However, landscape obtained data from eight transects that were subjected fragmentation and fire suppression have reduced fire to a winter burn in 1986 and a summer burn in 1997 and size and frequency (Myers 1990, Duncan and compared the recovery of the stand for the first five Schmalzer 2001). -

Tillandsia Recurvata Is the Most Wide
ZLATKO JANEBA Tillandsia recurvata illandsia recurvata is the most wide- even known to grow on roofs and power lines. spread bromeliad. It occurs in the T. recurvata is the type species of subgenus Dia- southern US, where it stretches phoranthema, which contains some 30 variable and from Florida all the way to Arizo- mostly miniature species that have small, incon- na, and as far south as as Argenti- spicuous flowers. Members of Diaphoranthema are na and Chile. It grows epiphytical- common and locally abundant in South Ameri- ly on trees, bushes, and cacti or as ca, with a distribution centered in Argentina and a petrophyte on rocky cliffs. It is Bolivia. Only two species reach North America: T. recurvata (aka Small Ballmoss) was found growing close to the ground on the side of the barrel cactus Echinocactus platyacanthus near La Ascención, Nuevo León, Mexico (right). More often, T. recurvata is spotted (right) clinging to the bark of pine trees (Pinus johannis and Pinus arizonica var stormiae), as seen here at a Tspot between Santa Lucia and El Pinito, Nuevo León. 2 CACTUS AND SUCCULENT JOURNAL T. recurvata, sometimes called Small Ballmoss, and Tillandsia species, such as T. capillaris, T. croca- T. usneoides, the well known Spanish Moss. ta, and T. mallemontii, which are found in simi- Polyploidy, the condition wherein a plant con- lar habitats but which have different flower char- tains more than one set of chromosomes in its acteristics. cells, is common in this subgenus. Normally we T. recurvata was described by Carl Linnaeus think of polyploidy resulting in larger-than-nor- as Renealmia recurvata in 1753, the same year mal plants, but these bromeliads tend to be quite that he erected the genus Tillandsia. -

Water Relations of Bromeliaceae in Their Evolutionary Context
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Apollo Botanical Journal of the Linnean Society, 2016, 181, 415–440. With 2 figures Think tank: water relations of Bromeliaceae in their evolutionary context JAMIE MALES* Department of Plant Sciences, University of Cambridge, Downing Street, Cambridge CB2 3EA, UK Received 31 July 2015; revised 28 February 2016; accepted for publication 1 March 2016 Water relations represent a pivotal nexus in plant biology due to the multiplicity of functions affected by water status. Hydraulic properties of plant parts are therefore likely to be relevant to evolutionary trends in many taxa. Bromeliaceae encompass a wealth of morphological, physiological and ecological variations and the geographical and bioclimatic range of the family is also extensive. The diversification of bromeliad lineages is known to be correlated with the origins of a suite of key innovations, many of which relate directly or indirectly to water relations. However, little information is known regarding the role of change in morphoanatomical and hydraulic traits in the evolutionary origins of the classical ecophysiological functional types in Bromeliaceae or how this role relates to the diversification of specific lineages. In this paper, I present a synthesis of the current knowledge on bromeliad water relations and a qualitative model of the evolution of relevant traits in the context of the functional types. I use this model to introduce a manifesto for a new research programme on the integrative biology and evolution of bromeliad water-use strategies. The need for a wide-ranging survey of morphoanatomical and hydraulic traits across Bromeliaceae is stressed, as this would provide extensive insight into structure– function relationships of relevance to the evolutionary history of bromeliads and, more generally, to the evolutionary physiology of flowering plants. -

Phylogeny and Biogeography of the American Live Oaks
Received Date : 03-Jan-2015 Revised Date : 15-May-2015 Accepted Date : 01-Jun-2015 Article type : Original Article Corresponding author email id: [email protected] Original Article Phylogeny and biogeography of the American live oaks (Quercus subsection Virentes): A genomic and population genetics approach Jeannine Cavender-Bares1* Antonio Gonzalez-Rodriguez2 Article Deren A.R. Eaton3 Andrew A. L. Hipp4,5 Anne Beulke1 Paul S. Manos6 Running head: Evolutionary history of the American live oaks 1Department of Ecology, Evolution and Behavior, University of Minnesota, Saint Paul, MN 55108 2Centro de Investigaciones en Ecosistemas, Universidad Nacional Autónoma de México, Morelia, Michoacán. 3Department of Ecology and Evolutionary Biology, Yale University, New Haven CT 4The Morton Arboretum, Lisle, Illinois 4The Field Museum, Chicago, Illinois 6Department of Biology, Duke University, Raleigh NC This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: Accepted 10.1111/mec.13269 This article is protected by copyright. All rights reserved. Keywords: Virentes, RADseq, genomic data, fossil calibration, phylogeography, introgression, ecological and climatic niches, Sea of Cortés, conservation Abstract The nature and timing of evolution of niche differentiation among closely related species remains an important question in ecology -

Northern Yellow Bat Roost Selection and Fidelity in South Carolina
Final Report South Carolina State Wildlife Grant SC T-F16AF00598 South Carolina Department of Natural Resources (SCDNR) May 1, 2016-June 30, 2017 Project Title: Northern Yellow Bat Roost Selection and Fidelity in South Carolina Mary Socci, Palmetto Bluff Conservancy (PBC), Jay Walea, PBC, Timothy White, PBC, Jason Robinson, Biological Systems Consultants, Inc., and Jennifer Kindel, SCDNR Objective 1: Radio-track healthy Northern yellow bats (≤ 10) captured by mist netting appropriate habitat in spring, summer, and fall 2016, ideally with at least 3 radio-tracking events in each season. Record roost switching, and describe roost sites selected. Accomplishments: Introduction: The primary purpose of this study was to investigate the roost site selection and fidelity of northern yellow bats (Lasiurus intermedius, syn. Dasypterus intermedius) by capturing, radio- tagging and tracking individual L. intermedius at Palmetto Bluff, a 15,000 acre, partially-developed tract in Beaufort County, South Carolina (Figures 1 and 2). Other objectives (2 and 3) were to obtain audio recordings of bats foraging in various habitats across the Palmetto Bluff property, including as many L. intermedius as possible and to initiate a public outreach program in order to educate the community on both the project and the environmental needs of bats, many of which are swiftly declining species in United States. Figure 1. Location of Palmetto Bluff 1 Figure 2. The Palmetto Bluff Development Tract The life history of northern yellow bats, a high-priority species in the Southeast, is poorly understood. Studies in coastal Georgia found that all yellow bats that were tracked roosted in Spanish moss in southern live oaks (Quercus virginiana) and sand live oaks (Quercus geminata) (Coleman et al. -

Wild Crop Relatives: Genomic and Breeding Resources: Forest Trees
Wild Crop Relatives: Genomic and Breeding Resources . Chittaranjan Kole Editor Wild Crop Relatives: Genomic and Breeding Resources Forest Trees Editor Prof. Chittaranjan Kole Director of Research Institute of Nutraceutical Research Clemson University 109 Jordan Hall Clemson, SC 29634 [email protected] ISBN 978-3-642-21249-9 e-ISBN 978-3-642-21250-5 DOI 10.1007/978-3-642-21250-5 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2011922649 # Springer-Verlag Berlin Heidelberg 2011 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: deblik, Berlin Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Dedication Dr. Norman Ernest Borlaug,1 the Father of Green Revolution, is well respected for his contribu- tions to science and society. There was or is not and never will be a single person on this Earth whose single-handed service to science could save millions of people from death due to starvation over a period of over four decades like Dr.