Molecular Characterization of the Diversity and Distribution of a Thermal Spring Microbial Community by Using Rrna and Metabolic Genesᰔ† Justine R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
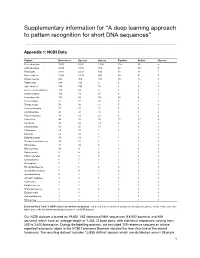
A Deep Learning Approach to Pattern Recognition for Short DNA Sequences”
Supplementary information for “A deep learning approach to pattern recognition for short DNA sequences” Appendix 1: NCBI Data Phylum References Species Genera Families Orders Classes Proteobacteria 7,053 5,061 1,106 158 55 9 Actinobacteria 4,768 3,313 383 68 29 6 Firmicutes 3,814 2,531 499 56 13 7 Bacteroidetes 1,934 1,525 360 39 8 7 Euryarchaeota 834 450 100 28 13 8 Tenericutes 266 195 8 5 4 1 Spirochaetes 146 100 16 6 4 1 Deinococcus-Thermus 118 99 9 3 2 1 Crenarchaeota 113 61 27 8 5 1 Cyanobacteria 113 88 59 30 8 2 Fusobacteria 75 37 10 2 1 1 Thermotogae 70 48 13 5 4 1 Verrucomicrobia 57 53 22 7 4 3 Acidobacteria 45 41 19 5 5 4 Planctomycetes 44 31 23 5 3 2 Chloroflexi 44 35 26 15 12 8 Aquificae 43 32 14 4 2 1 Synergistetes 31 25 15 1 1 1 Chlamydiae 28 18 7 5 2 1 Chlorobi 21 16 5 1 1 1 Deferribacteres 15 11 7 1 1 1 Thermodesulfobacteria 14 12 5 1 1 1 Nitrospirae 11 10 3 1 1 1 Fibrobacteres 10 4 3 3 3 3 Balneolaeota 9 9 4 1 1 1 Chrysiogenetes 6 4 3 1 1 1 Lentisphaerae 6 5 3 3 3 2 Dictyoglomi 5 2 1 1 1 1 Rhodothermaeota 5 5 3 2 1 1 Gemmatimonadetes 5 4 3 2 2 2 Ignavibacteriae 4 2 2 2 1 1 Armatimonadetes 4 3 3 3 3 3 Caldiserica 3 1 1 1 1 1 Calditrichaeota 2 2 1 1 1 1 Thaumarchaeota 2 2 2 2 2 2 Elusimicrobia 2 1 1 1 1 1 Kiritimatiellaeota 1 1 1 1 1 1 Nitrospinae 1 1 1 1 1 1 Extended Data Table 1: NCBI dataset breakdown by phylum. -

7. References
University of Akureyri Department of Natural Resource Science 7. References Ammann, E. C., Reed, L. L., & Durichek, J. J. (1968). Gas consumptions and Growth Rate of Hydrogenomonas eutropha in Continuous Culture. Applied Microbiology , 16, (6), 822-826. Aguiar, P., Beveridge, T. J., & Reysenbach, A.-L. (2004). Sulfurihydrogenibium azorense, sp. nov., a thermophilic hydrogen oxidizing microaerophile from terrestrial hot springs in the Azores. International Journal of Systematic and Evolutionary Microbiology , 54, 33-39. Altschul, S., Gish, W., Miller, W., Myers, E., & Lipman, D. (1990). "Basic local alignment search tool.". J. Mol. Biol. , 215:403-410. Amend, J., & Shock, E. (2001). Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiology Reviews , 25, 175-243. Aragno, M. (1978). Enrichment, isolation and preliminary characterization of a thermophilic, endospore-forming hydrogen bacterium. FEMS Micobiol. Lett. , 3: 13-15. Aragno, M. (1992). The Thermophilic, Aerobic, Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Trüper, M. Dworkin, W. Harder, & K. Schleifer, The Prokaryotes, a handbook on biology of bacteria. 2nd ed. vol. 4 (pp. 3917-3933.). New York: Springer Verlag. Aragno, M., & Schlegel, H. G. (1992). The mesophilic Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Truper, M. Dworkin, W. Harder, & K.-H. Schleifer, The Prokaryotes 2nd. ed. (pp. 344-384). New York: Springer. Ármannson, H. (2002, May 30-31). Erindi á ráðstefnu um málefni veitufyrirtækja . Grænt bókhald í jarðhita- samanburður á útblæstri við aðra orkugjafa . Akureyri, Iceland: Samorka. Bae, S., Kwak, K., Kim, S., Chung, S., & Igarashi, Y. (2001). Isolation and Characterization of CO2-Fixing Hydrogen -Oxidizing Marine 109 University of Akureyri Department of Natural Resource Science Bacteria. -

Characterization of the Uncommon Enzymes from (2004)
OPEN Mannosylglucosylglycerate biosynthesis SUBJECT AREAS: in the deep-branching phylum WATER MICROBIOLOGY MARINE MICROBIOLOGY Planctomycetes: characterization of the HOMEOSTASIS MULTIENZYME COMPLEXES uncommon enzymes from Rhodopirellula Received baltica 13 March 2013 Sofia Cunha1, Ana Filipa d’Avo´1, Ana Mingote2, Pedro Lamosa3, Milton S. da Costa1,4 & Joana Costa1,4 Accepted 23 July 2013 1Center for Neuroscience and Cell Biology, University of Coimbra, 3004-517 Coimbra, Portugal, 2Instituto de Tecnologia Quı´mica Published e Biolo´gica, Universidade Nova de Lisboa, 2780-157 Oeiras, Portugal, 3Centro de Ressonaˆncia Magne´tica Anto´nio Xavier, 7 August 2013 Instituto de Tecnologia Quı´mica e Biolo´gica, Universidade Nova de Lisboa, 2781-901 Oeiras, Portugal, 4Department of Life Sciences, University of Coimbra, Apartado 3046, 3001-401 Coimbra, Portugal. Correspondence and The biosynthetic pathway for the rare compatible solute mannosylglucosylglycerate (MGG) accumulated by requests for materials Rhodopirellula baltica, a marine member of the phylum Planctomycetes, has been elucidated. Like one of the should be addressed to pathways used in the thermophilic bacterium Petrotoga mobilis, it has genes coding for J.C. ([email protected].) glucosyl-3-phosphoglycerate synthase (GpgS) and mannosylglucosyl-3-phosphoglycerate (MGPG) synthase (MggA). However, unlike Ptg. mobilis, the mesophilic R. baltica uses a novel and very specific MGPG phosphatase (MggB). It also lacks a key enzyme of the alternative pathway in Ptg. mobilis – the mannosylglucosylglycerate synthase (MggS) that catalyses the condensation of glucosylglycerate with GDP-mannose to produce MGG. The R. baltica enzymes GpgS, MggA, and MggB were expressed in E. coli and characterized in terms of kinetic parameters, substrate specificity, temperature and pH dependence. -

And Thermo-Adaptation in Hyperthermophilic Archaea: Identification of Compatible Solutes, Accumulation Profiles, and Biosynthetic Routes in Archaeoglobus Spp
Universidade Nova de Lisboa Osmo- andInstituto thermo de Tecnologia-adaptation Química e Biológica in hyperthermophilic Archaea: Subtitle Subtitle Luís Pedro Gafeira Gonçalves Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. OH OH OH CDP c c c - CMP O O - PPi O3P P CTP O O O OH OH OH OH OH OH O- C C C O P O O P i Dissertation presented to obtain the Ph.D degree in BiochemistryO O- Instituto de Tecnologia Química e Biológica | Universidade Nova de LisboaP OH O O OH OH OH Oeiras, Luís Pedro Gafeira Gonçalves January, 2008 2008 Universidade Nova de Lisboa Instituto de Tecnologia Química e Biológica Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. This dissertation was presented to obtain a Ph. D. degree in Biochemistry at the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa. By Luís Pedro Gafeira Gonçalves Supervised by Prof. Dr. Helena Santos Oeiras, January, 2008 Apoio financeiro da Fundação para a Ciência e Tecnologia (POCI 2010 – Formação Avançada para a Ciência – Medida IV.3) e FSE no âmbito do Quadro Comunitário de apoio, Bolsa de Doutoramento com a referência SFRH / BD / 5076 / 2001. ii ACKNOWNLEDGMENTS The work presented in this thesis, would not have been possible without the help, in terms of time and knowledge, of many people, to whom I am extremely grateful. Firstly and mostly, I need to thank my supervisor, Prof. Helena Santos, for her way of thinking science, her knowledge, her rigorous criticism, and her commitment to science. -

Molecular Evolution of the Oxygen-Binding Hemerythrin Domain
RESEARCH ARTICLE Molecular Evolution of the Oxygen-Binding Hemerythrin Domain Claudia Alvarez-Carreño1, Arturo Becerra1, Antonio Lazcano1,2* 1 Facultad de Ciencias, Universidad Nacional Autónoma de México, Apdo. Postal 70–407, Cd. Universitaria, 04510, Mexico City, Mexico, 2 Miembro de El Colegio Nacional, Ciudad de México, México * [email protected] a11111 Abstract Background The evolution of oxygenic photosynthesis during Precambrian times entailed the diversifica- tion of strategies minimizing reactive oxygen species-associated damage. Four families of OPEN ACCESS oxygen-carrier proteins (hemoglobin, hemerythrin and the two non-homologous families of Citation: Alvarez-Carreño C, Becerra A, Lazcano A arthropodan and molluscan hemocyanins) are known to have evolved independently the (2016) Molecular Evolution of the Oxygen-Binding Hemerythrin Domain. PLoS ONE 11(6): e0157904. capacity to bind oxygen reversibly, providing cells with strategies to cope with the evolution- doi:10.1371/journal.pone.0157904 ary pressure of oxygen accumulation. Oxygen-binding hemerythrin was first studied in Editor: Nikolas Nikolaidis, California State University marine invertebrates but further research has made it clear that it is present in the three Fullerton, UNITED STATES domains of life, strongly suggesting that its origin predated the emergence of eukaryotes. Received: April 5, 2016 Accepted: June 7, 2016 Results Published: June 23, 2016 Oxygen-binding hemerythrins are a monophyletic sub-group of the hemerythrin/HHE (histi- dine, histidine, glutamic acid) cation-binding domain. Oxygen-binding hemerythrin homo- Copyright: © 2016 Alvarez-Carreño et al. This is an open access article distributed under the terms of the logs were unambiguously identified in 367/2236 bacterial, 21/150 archaeal and 4/135 Creative Commons Attribution License, which permits eukaryotic genomes. -

NOC04013 Application.Pdf(PDF, 453
ER-AN-02N 10/02 Application for approval to import into FORM 2N containment any new organism that is not genetically modified, under Section 40 of the Page 1 Hazardous Substances and New Organisms Act 1996 FORM NO2N Application for approval to IMPORT INTO CONTAINMENT ANY NEW ORGANISM THAT IS NOT GENETICALLY MODIFIED under section 40 of the Hazardous Substances and New Organisms Act 1996 Application Title: Importation of sediment/water samples collected from hydrothermal seamounts outside New Zealand Territorial waters containing Extremophilic microorganisms Applicant Organisation: Institute of Geological & Nuclear Sciences ERMA Office use only Application Code: Formally received:____/____/____ ERMA NZ Contact: Initial Fee Paid: $ Application Status: ER-AN-02N 10/02 Application for approval to import into FORM 2N containment any new organism that is not genetically modified, under Section 40 of the Page 2 Hazardous Substances and New Organisms Act 1996 IMPORTANT 1. An associated User Guide is available for this form. You should read the User Guide before completing this form. If you need further guidance in completing this form please contact ERMA New Zealand. 2. This application form covers importation into containment of any new organism that is not genetically modified, under section 40 of the Act. 3. If you are making an application to import into containment a genetically modified organism you should complete Form NO2G, instead of this form (Form NO2N). 4. This form, together with form NO2G, replaces all previous versions of Form 2. Older versions should not now be used. You should periodically check with ERMA New Zealand or on the ERMA New Zealand web site for new versions of this form. -

BEING Aquifex Aeolicus: UNTANGLING a HYPERTHERMOPHILE‘S CHECKERED PAST
BEING Aquifex aeolicus: UNTANGLING A HYPERTHERMOPHILE‘S CHECKERED PAST by Robert J.M. Eveleigh Submitted in partial fulfillment of the requirements for the degree of Master of Science at Dalhousie University Halifax, Nova Scotia December 2011 © Copyright by Robert J.M. Eveleigh, 2011 DALHOUSIE UNIVERSITY DEPARTMENT OF COMPUTATIONAL BIOLOGY AND BIOINFORMATICS The undersigned hereby certify that they have read and recommend to the Faculty of Graduate Studies for acceptance a thesis entitled ―BEING Aquifex aeolicus: UNTANGLING A HYPERTHERMOPHILE‘S CHECKERED PAST‖ by Robert J.M. Eveleigh in partial fulfillment of the requirements for the degree of Master of Science. Dated: December 13, 2011 Co-Supervisors: _________________________________ _________________________________ Readers: _________________________________ ii DALHOUSIE UNIVERSITY DATE: December 13, 2011 AUTHOR: Robert J.M. Eveleigh TITLE: BEING Aquifex aeolicus: UNTANGLING A HYPERTHERMOPHILE‘S CHECKERED PAST DEPARTMENT OR SCHOOL: Department of Computational Biology and Bioinformatics DEGREE: MSc CONVOCATION: May YEAR: 2012 Permission is herewith granted to Dalhousie University to circulate and to have copied for non-commercial purposes, at its discretion, the above title upon the request of individuals or institutions. I understand that my thesis will be electronically available to the public. The author reserves other publication rights, and neither the thesis nor extensive extracts from it may be printed or otherwise reproduced without the author‘s written permission. The author attests that permission has been obtained for the use of any copyrighted material appearing in the thesis (other than the brief excerpts requiring only proper acknowledgement in scholarly writing), and that all such use is clearly acknowledged. _______________________________ Signature of Author iii TABLE OF CONTENTS List of Tables .................................................................................................................... -

Marinitoga Lauensis Sp. Nov., a Novel Deep-Sea Hydrothermal Vent Thermophilic Anaerobic Heterotroph with a Prophage
Portland State University PDXScholar Biology Faculty Publications and Presentations Biology 5-1-2019 Marinitoga Lauensis sp. nov., A Novel Deep-sea Hydrothermal Vent Thermophilic Anaerobic Heterotroph with a Prophage Stéphane L'Haridon Univ Brest, CNRS, IFREMER Léna Gouhie CNRS, IFREMER Emily St. John Portland State University, [email protected] Anna-Louise Reysenbach Portland State University, [email protected] Follow this and additional works at: https://pdxscholar.library.pdx.edu/bio_fac Let us know how access to this document benefits ou.y Citation Details Published as: L’Haridon, S., Gouhier, L., John, E. S., & Reysenbach, A. L. (2019). Marinitoga lauensis sp. nov., a novel deep-sea hydrothermal vent thermophilic anaerobic heterotroph with a prophage. Systematic and applied microbiology, 42(3), 343-347. This Post-Print is brought to you for free and open access. It has been accepted for inclusion in Biology Faculty Publications and Presentations by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Accepted Manuscript Title: Marinitoga lauensis sp. nov., a novel deep-sea hydrothermal vent thermophilic anaerobic heterotroph with a prophage Authors: Stephane´ L’Haridon, Lena´ Gouhier, Emily St. John, Anna-Louise Reysenbach PII: S0723-2020(18)30493-4 DOI: https://doi.org/10.1016/j.syapm.2019.02.006 Reference: SYAPM 25981 To appear in: Received date: 30 November 2018 Revised date: 17 February 2019 Accepted date: 26 February 2019 Please cite this article as: Stephane´ L’Haridon, Lena´ Gouhier, Emily St.John, Anna- Louise Reysenbach, Marinitoga lauensis sp.nov., a novel deep-sea hydrothermal vent thermophilic anaerobic heterotroph with a prophage, Systematic and Applied Microbiology https://doi.org/10.1016/j.syapm.2019.02.006 This is a PDF file of an unedited manuscript that has been accepted for publication. -

A Method for Achieving Complete Microbial Genomes and Improving Bins from Metagenomics Data
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.05.979740; this version posted July 18, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 A method for achieving complete microbial genomes and improving 2 bins from metagenomics data 3 4 Authors: 5 Lauren M. Lui1, Torben N. Nielsen1, Adam P. Arkin1,2,3* 6 7 Affiliations: 8 1Environmental Genomics and Systems Biology Division, Lawrence Berkeley National 9 Laboratory, Berkeley, CA, USA. 10 2Department of Bioengineering, University of California, Berkeley, CA, USA 11 3Innovative Genomics Institute, Berkeley, CA, USA 12 13 *Correspondence: [email protected] 14 15 16 17 18 19 20 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.03.05.979740; this version posted July 18, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 22 Abstract 23 Metagenomics facilitates the study of the genetic information from uncultured microbes 24 and complex microbial communities. Assembling complete microbial genomes (i.e., 25 circular with no misassemblies) from metagenomics data is difficult because most 26 samples have high organismal complexity and strain diversity. Less than 100 27 circularized bacterial and archaeal genomes have been assembled from metagenomics 28 data despite the thousands of datasets that are available. -

Distribution of Aerobic Arsenite Oxidase Genes Within the Aquificales
Interdisciplinary Studies on Environmental Chemistry — Biological Responses to Contaminants, Eds., N. Hamamura, S. Suzuki, S. Mendo, C. M. Barroso, H. Iwata and S. Tanabe, pp. 47–55. © by TERRAPUB, 2010. Distribution of Aerobic Arsenite Oxidase Genes within the Aquificales Natsuko HAMAMURA1,2, Rich E. MACUR3, Yitai LIU2, William P. INSKEEP3 and Anna-Louise REYSENBACH2 1Center for Marine Environmental Studies, Ehime University, Matsuyama 790-8577 Japan 2Department of Biology, Portland State University, Portland, OR 97201 U.S.A. 3Thermal Biology Institute and Department of Land Resources and Environmental Sciences, Montana State University, Bozeman, MT 59717 U.S.A. (Received 4 January 2010; accepted 25 January 2010) Abstract—The Aquificales are one of the dominant bacterial orders associated with arsenic-rich geothermal environments, however, their role in arsenic transformations has not been fully characterized. In this report, we examined the distribution of aerobic arsenite oxidase genes (aroA-like) among 26 Aquificales isolates from geographically distinct marine and terrestrial hydrothermal systems. Arsenite-oxidase genes were detected in two Hydrogenobacter strains and one Sulfurihydrogenibium strain isolated from terrestrial springs in the Uzon Caldera and the Geyser Valley of Kamchatka, Russia, but were absent in other phylogenetically closely related strains isolated from the same thermal systems. The arsenite-oxidation activities of these aroA-positive strains were also confirmed. The newly identified aroA- like sequences share high sequence similarity to aroA genes identified in other thermophilic bacteria, but still formed separate clades from previously identified aroA sequences. These results extend our knowledge of the diversity and distribution of Mo-pterin arsenite oxidases in the Aquificales, an important step in understanding the evolutionary relationships of deeply-rooted bacterial arsenite oxidases relative to the diversity of aroA-like genes now known to exist across the bacterial domain. -

Alpha-Carbonic Anhydrases from Hydrothermal Vent Sources As Potential Carbon Dioxide Sequestration Agents: in Silico Sequence, Structure and Dynamics Analyses
International Journal of Molecular Sciences Article Alpha-Carbonic Anhydrases from Hydrothermal Vent Sources as Potential Carbon Dioxide Sequestration Agents: In Silico Sequence, Structure and Dynamics Analyses Colleen Varaidzo Manyumwa 1 , Reza Zolfaghari Emameh 2 and Özlem Tastan Bishop 1,* 1 Research Unit in Bioinformatics (RUBi), Department of Biochemistry and Microbiology, Rhodes University, Makhanda/Grahamstown 6140, South Africa; [email protected] 2 Department of Energy and Environmental Biotechnology, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran 14965/161, Iran; [email protected] * Correspondence: [email protected]; Tel.: +27-46-603-8072; Fax: +27-46-603-7576 Received: 28 September 2020; Accepted: 27 October 2020; Published: 29 October 2020 Abstract: With the increase in CO2 emissions worldwide and its dire effects, there is a need to reduce CO2 concentrations in the atmosphere. Alpha-carbonic anhydrases (α-CAs) have been identified as suitable sequestration agents. This study reports the sequence and structural analysis of 15 α-CAs from bacteria, originating from hydrothermal vent systems. Structural analysis of the multimers enabled the identification of hotspot and interface residues. Molecular dynamics simulations of the homo-multimers were performed at 300 K, 363 K, 393 K and 423 K to unearth potentially thermostable α-CAs. Average betweenness centrality (BC) calculations confirmed the relevance of some hotspot and interface residues. The key residues responsible for dimer thermostability were identified by comparing fluctuating interfaces with stable ones, and were part of conserved motifs. Crucial long-lived hydrogen bond networks were observed around residues with high BC values. Dynamic cross correlation fortified the relevance of oligomerization of these proteins, thus the importance of simulating them in their multimeric forms. -

Thermocrinis Jamiesonii Sp. Nov., a Thiosulfate-Oxidizing, Autotropic Thermophile Isolated from a Geothermal Spring Jeremy A
International Journal of Systematic and Evolutionary Microbiology (2015), 65, 4769–4775 DOI 10.1099/ijsem.0.000647 Thermocrinis jamiesonii sp. nov., a thiosulfate-oxidizing, autotropic thermophile isolated from a geothermal spring Jeremy A. Dodsworth,1,2 John C. Ong,2 Amanda J. Williams,23 Alice C. Dohnalkova3 and Brian P. Hedlund2 Correspondence 1Department of Biology, California State University, San Bernardino, CA 92407, USA Jeremy A. Dodsworth 2School of Life Sciences, University of Nevada, Las Vegas, NV 89154, USA [email protected] 3Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 99352, USA An obligately thermophilic, chemolithotrophic, microaerophilic bacterium, designated strain GBS1T, was isolated from the water column of Great Boiling Spring, Nevada, USA. Thiosulfate was required for growth. Although capable of autotrophy, growth of GBS1T was enhanced in the presence of acetate, peptone or Casamino acids. Growth occurred at 70–85 8C with an optimum at 80 8C, at pH 6.50–7.75 with an optimum at pH 7.25, with 0.5–8 % oxygen with an optimum at 1–2 % and with j200 mM NaCl. The doubling time under optimal growth 2 conditions was 1.3 h, with a final mean cell density of 6.2¡0.56107 cells ml 1. Non-motile, rod-shaped cells 1.4–2.460.4–0.6 mm in size occurred singly or in pairs. The major cellular fatty acids (.5 % of the total) were C20 : 1v9c,C18 : 0,C16 : 0 and C20 : 0. Phylogenetic analysis of the GBS1T 16S rRNA gene sequence indicated an affiliation with Thermocrinis ruber and other species of the genus Thermocrinis, but determination of 16S rRNA gene sequence similarity (j97.10 %) and in silico estimated DNA–DNA hybridization values (j18.4 %) with the type strains of recognized Thermocrinis species indicate that the novel strain is distinct from described species.