And Thermo-Adaptation in Hyperthermophilic Archaea: Identification of Compatible Solutes, Accumulation Profiles, and Biosynthetic Routes in Archaeoglobus Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Opportunities Revealed by Biotechnological Explorations of Extremophiles - Mircea Podar and Anna-Louise Reysenbach
BIOTECHNOLOGY – Vol .III – New Opportunities Revealed by Biotechnological Explorations of Extremophiles - Mircea Podar and Anna-Louise Reysenbach NEW OPPORTUNITIES REVEALED BY BIOTECHNOLOGICAL EXPLORATIONS OF EXTREMOPHILES Mircea Podar and Anna-Louise Reysenbach Department of Biology, Portland State University, Portland, OR 97201, USA. Keywords: extremophiles, genomics, biotechnology, enzymes, metagenomics. Contents 1. Introduction 2. Extremophiles and Biomolecules 3. Extremophile Genomics Exposing the Biotechnological Potential 4. Tapping into the Hidden Biotechnological Potential through Metagenomics 5. Unexplored Frontiers and Future Prospects Acknowledgements Glossary Bibliography Biographical Sketches Summary Over the past few decades the extremes at which life thrives has continued to challenge our understanding of biochemistry, biology and evolution. As more new extremophiles are brought into laboratory culture, they have provided a multitude of new potential applications for biotechnology. Furthermore, more recently, innovative culturing approaches, environmental genome sequencing and whole genome sequencing have provided new opportunities for biotechnological exploration of extremophiles. 1. Introduction Organisms that live at the extremes of pH (>pH 8.5,< pH 5.0), temperature (>45°C, <15°C), pressure (>500 atm), salinity (>1.0M NaCl) and in high concentrations of recalcitrant substances or heavy metals (extremophiles) represent one of the last frontiers for biotechnological and industrial discovery. As we learn more about the -

Extremozymes of the Hot and Salty Halothermothrix Orenii
Extremozymes of the Hot and Salty Halothermothrix orenii Author Kori, Lokesh D Published 2012 Thesis Type Thesis (PhD Doctorate) School School of Biomolecular and Physical Sciences DOI https://doi.org/10.25904/1912/2191 Copyright Statement The author owns the copyright in this thesis, unless stated otherwise. Downloaded from http://hdl.handle.net/10072/366220 Griffith Research Online https://research-repository.griffith.edu.au Extremozymes of the hot and salty Halothermothrix orenii LOKESH D. KORI (M.Sc. Biotechnology) School of Biomolecular and Physical Sciences Science, Environment, Engineering and Technology Griffith University, Australia Submitted in fulfillment of the requirements of the degree of Doctor of Philosophy December 2011 STATEMENT OF ORIGINALITY STATEMENT OF ORIGINALITY This work has not previously been submitted for a degree or diploma in any university. To the best of my knowledge and belief, the thesis contains no material previously published or written by another person except where due reference is made in the thesis itself. LOKESH DULICHAND KORI II ACKNOWLEDGEMENTS ACKNOWLEDGEMENTS I owe my deepest gratitude to my supervisor Prof. Bharat Patel, for offering me an opportunity for being his postgraduate. His boundless knowledge motivates me for keep going and enjoy the essence of science. Without his guidance, great patience and advice, I could not finish my PhD program successfully. I take this opportunity to give my heartiest thanks to Assoc. Prof. Andreas Hofmann, (Structural Chemistry, Eskitis Institute for Cell & Molecular Therapies, Griffith University) for his support and encouragement for crystallographic work. I am grateful to him for teaching me about the protein structures, in silico analysis and their hidden chemistry. -

Methanothermus Fervidus Type Strain (V24S)
UC Davis UC Davis Previously Published Works Title Complete genome sequence of Methanothermus fervidus type strain (V24S). Permalink https://escholarship.org/uc/item/9367m39j Journal Standards in genomic sciences, 3(3) ISSN 1944-3277 Authors Anderson, Iain Djao, Olivier Duplex Ngatchou Misra, Monica et al. Publication Date 2010-11-20 DOI 10.4056/sigs.1283367 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Standards in Genomic Sciences (2010) 3:315-324 DOI:10.4056/sigs.1283367 Complete genome sequence of Methanothermus fervidus type strain (V24ST) Iain Anderson1, Olivier Duplex Ngatchou Djao2, Monica Misra1,3, Olga Chertkov1,3, Matt Nolan1, Susan Lucas1, Alla Lapidus1, Tijana Glavina Del Rio1, Hope Tice1, Jan-Fang Cheng1, Roxanne Tapia1,3, Cliff Han1,3, Lynne Goodwin1,3, Sam Pitluck1, Konstantinos Liolios1, Natalia Ivanova1, Konstantinos Mavromatis1, Natalia Mikhailova1, Amrita Pati1, Evelyne Brambilla4, Amy Chen5, Krishna Palaniappan5, Miriam Land1,6, Loren Hauser1,6, Yun-Juan Chang1,6, Cynthia D. Jeffries1,6, Johannes Sikorski4, Stefan Spring4, Manfred Rohde2, Konrad Eichinger7, Harald Huber7, Reinhard Wirth7, Markus Göker4, John C. Detter1, Tanja Woyke1, James Bristow1, Jonathan A. Eisen1,8, Victor Markowitz5, Philip Hugenholtz1, Hans-Peter Klenk4, and Nikos C. Kyrpides1* 1 DOE Joint Genome Institute, Walnut Creek, California, USA 2 HZI – Helmholtz Centre for Infection Research, Braunschweig, Germany 3 Los Alamos National Laboratory, Bioscience Division, Los Alamos, New Mexico, USA 4 DSMZ - German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany 5 Biological Data Management and Technology Center, Lawrence Berkeley National Laboratory, Berkeley, California, USA 6 Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA 7 University of Regensburg, Archaeenzentrum, Regensburg, Germany 8 University of California Davis Genome Center, Davis, California, USA *Corresponding author: Nikos C. -

The Genome of Prasinoderma Coloniale Unveils the Existence of a Third Phylum Within Green Plants
SUPPLEMENTARY INFORMATIONARTICLES https://doi.org/10.1038/s41559-020-1221-7 In the format provided by the authors and unedited. The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants Linzhou Li1,2,13, Sibo Wang1,3,13, Hongli Wang1,4, Sunil Kumar Sahu 1, Birger Marin 5, Haoyuan Li1, Yan Xu1,4, Hongping Liang1,4, Zhen Li 6, Shifeng Cheng1, Tanja Reder5, Zehra Çebi5, Sebastian Wittek5, Morten Petersen3, Barbara Melkonian5,7, Hongli Du8, Huanming Yang1, Jian Wang1, Gane Ka-Shu Wong 1,9, Xun Xu 1,10, Xin Liu 1, Yves Van de Peer 6,11,12 ✉ , Michael Melkonian5,7 ✉ and Huan Liu 1,3 ✉ 1State Key Laboratory of Agricultural Genomics, BGI-Shenzhen, Shenzhen, China. 2Department of Biotechnology and Biomedicine, Technical University of Denmark, Lyngby, Denmark. 3Department of Biology, University of Copenhagen, Copenhagen, Denmark. 4BGI Education Center, University of Chinese Academy of Sciences, Shenzhen, China. 5Institute for Plant Sciences, Department of Biological Sciences, University of Cologne, Cologne, Germany. 6Department of Plant Biotechnology and Bioinformatics (Ghent University) and Center for Plant Systems Biology, Ghent, Belgium. 7Central Collection of Algal Cultures, Faculty of Biology, University of Duisburg-Essen, Essen, Germany. 8School of Biology and Biological Engineering, South China University of Technology, Guangzhou, China. 9Department of Biological Sciences and Department of Medicine, University of Alberta, Edmonton, Alberta, Canada. 10Guangdong Provincial Key Laboratory of Genome Read and Write, BGI-Shenzhen, Shenzhen, China. 11College of Horticulture, Nanjing Agricultural University, Nanjing, China. 12Centre for Microbial Ecology and Genomics, Department of Biochemistry, Genetics and Microbiology, University of Pretoria, Pretoria, South Africa. -
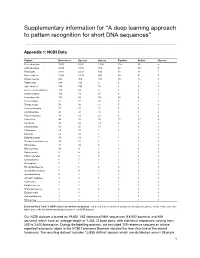
A Deep Learning Approach to Pattern Recognition for Short DNA Sequences”
Supplementary information for “A deep learning approach to pattern recognition for short DNA sequences” Appendix 1: NCBI Data Phylum References Species Genera Families Orders Classes Proteobacteria 7,053 5,061 1,106 158 55 9 Actinobacteria 4,768 3,313 383 68 29 6 Firmicutes 3,814 2,531 499 56 13 7 Bacteroidetes 1,934 1,525 360 39 8 7 Euryarchaeota 834 450 100 28 13 8 Tenericutes 266 195 8 5 4 1 Spirochaetes 146 100 16 6 4 1 Deinococcus-Thermus 118 99 9 3 2 1 Crenarchaeota 113 61 27 8 5 1 Cyanobacteria 113 88 59 30 8 2 Fusobacteria 75 37 10 2 1 1 Thermotogae 70 48 13 5 4 1 Verrucomicrobia 57 53 22 7 4 3 Acidobacteria 45 41 19 5 5 4 Planctomycetes 44 31 23 5 3 2 Chloroflexi 44 35 26 15 12 8 Aquificae 43 32 14 4 2 1 Synergistetes 31 25 15 1 1 1 Chlamydiae 28 18 7 5 2 1 Chlorobi 21 16 5 1 1 1 Deferribacteres 15 11 7 1 1 1 Thermodesulfobacteria 14 12 5 1 1 1 Nitrospirae 11 10 3 1 1 1 Fibrobacteres 10 4 3 3 3 3 Balneolaeota 9 9 4 1 1 1 Chrysiogenetes 6 4 3 1 1 1 Lentisphaerae 6 5 3 3 3 2 Dictyoglomi 5 2 1 1 1 1 Rhodothermaeota 5 5 3 2 1 1 Gemmatimonadetes 5 4 3 2 2 2 Ignavibacteriae 4 2 2 2 1 1 Armatimonadetes 4 3 3 3 3 3 Caldiserica 3 1 1 1 1 1 Calditrichaeota 2 2 1 1 1 1 Thaumarchaeota 2 2 2 2 2 2 Elusimicrobia 2 1 1 1 1 1 Kiritimatiellaeota 1 1 1 1 1 1 Nitrospinae 1 1 1 1 1 1 Extended Data Table 1: NCBI dataset breakdown by phylum. -

The Status of Natural Resources on the High-Seas
The status of natural resources on the high-seas Part 1: An environmental perspective Part 2: Legal and political considerations An independent study conducted by: The Southampton Oceanography Centre & Dr. A. Charlotte de Fontaubert The status of natural resources on the high-seas i The status of natural resources on the high-seas Published May 2001 by WWF-World Wide Fund for Nature (Formerly World Wildlife Fund) Gland, Switzerland. Any reproduction in full or in part of this publication must mention the title and credit the above mentioned publisher as the copyright owner. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of WWF or IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of WWF or IUCN. Published by: WWF International, Gland, Switzerland IUCN, Gland, Switzerland and Cambridge, UK. Copyright: © text 2001 WWF © 2000 International Union for Conservation of Nature and Natural Resources © All photographs copyright Southampton Oceanography Centre Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: WWF/IUCN (2001). The status of natural resources on the high-seas. WWF/IUCN, Gland, Switzerland. Baker, C.M., Bett, B.J., Billett, D.S.M and Rogers, A.D. -

A Proposal to Rename the Hyperthermophile Pyrococcus Woesei As Pyrococcus Furiosus Subsp
Archaea 1, 277–283 © 2004 Heron Publishing—Victoria, Canada A proposal to rename the hyperthermophile Pyrococcus woesei as Pyrococcus furiosus subsp. woesei WIROJNE KANOKSILAPATHAM,1 JUAN M. GONZÁLEZ,1,2 DENNIS L. MAEDER,1 1,3 1,4 JOCELYNE DIRUGGIERO and FRANK T. ROBB 1 Center of Marine Biotechnology, University of Maryland Biotechnology Institute, Baltimore, MD 21202, USA 2 Present address: IRNAS-CSIC, P.O. Box 1052, 41080 Sevilla, Spain 3 Department of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20274, USA 4 Corresponding author ([email protected]) Received April 8, 2004; accepted July 28, 2004; published online August 31, 2004 Summary Pyrococcus species are hyperthermophilic mem- relatively rapidly at temperatures above 90 °C and produce ° bers of the order Thermococcales, with optimal growth tem- H2S from elemental sulfur (S ) (Zillig et al. 1987). Two Pyro- peratures approaching 100 °C. All species grow heterotrophic- coccus species, P. furiosus (Fiala and Stetter 1986) and ° ally and produce H2 or, in the presence of elemental sulfur (S ), P. woesei (Zillig et al. 1987), were isolated from marine sedi- H2S. Pyrococcus woesei and P.furiosus were isolated from ma- ments at a Vulcano Island beach site in Italy. They have similar rine sediments at the same Vulcano Island beach site and share morphological and physiological characteristics: both species many morphological and physiological characteristics. We re- are cocci and move by means of a tuft of polar flagella. They port here that the rDNA operons of these strains have identical are heterotrophic and grow optimally at 95–100 °C, utilizing sequences, including their intergenic spacer regions and part of peptides as major carbon and nitrogen sources. -

Adaptin, a Component of Clathrin-Coated Vesicles Associated with the Golgi Apparatus
Cloning and Expression of -Adaptin, a Component of Clathrin-coated Vesicles Associated with the Golgi Apparatus Margaret S. Robinson Department of Clinical Biochemistry, University of Cambridge, Addenbrooke's Hospital, Cambridge CB2 2QR England Abstract. Adaptins are the major components of all. Weaker homology was seen between ~/- and adaptors, the protein complexes that link clathrin to /~-adaptins. Like both a- and #-adaptins, 3,-adaptin has transmembrane proteins (e.g., receptors) in coated pits a proline and glycine-rich hinge region, dividing it and vesicles. The plasma membrane adaptor contains into NH2- and COOH-terminal domains. A chimeric an ot-adaptin subunit and a/~-adaptin subunit, while 3,-adaptin was constructed from the mouse and bovine the Golgi adaptor contains a 3,-adaptin subunit and a cDNAs and transfected into Rat 1 fibroblasts. Im- /~'-adaptin subunit. A partial cDNA clone encoding munofluorescence microscopy was carded out using an 3,-adaptin was isolated from a bovine brain expression mAb which recognizes an epitope present on the chi- library by screening with antibodies, and was used to mera but not found on the rodent protein. The con- obtain a cDNA clone from a mouse brain library con- struct was found to have a distribution typical of en- taining the full coding sequence. The identity of the dogenous "¥-adaptin. Using this transfection system, it clones was confirmed by protein sequencing. The should now be possible to exchange domains between deduced amino acid sequence of 3,-adaptin was found or- and "r-adaptins, to try to find out how adaptors are to be homologous to that of c~-adaptin, with several targeted to the appropriate membrane compartment of stretches of identical amino acids or conservative sub- the cell, and how they recruit the appropriate recep- stitutions in the first *70 kD, and 25 % identity over- tors into the coated vesicle. -

7. References
University of Akureyri Department of Natural Resource Science 7. References Ammann, E. C., Reed, L. L., & Durichek, J. J. (1968). Gas consumptions and Growth Rate of Hydrogenomonas eutropha in Continuous Culture. Applied Microbiology , 16, (6), 822-826. Aguiar, P., Beveridge, T. J., & Reysenbach, A.-L. (2004). Sulfurihydrogenibium azorense, sp. nov., a thermophilic hydrogen oxidizing microaerophile from terrestrial hot springs in the Azores. International Journal of Systematic and Evolutionary Microbiology , 54, 33-39. Altschul, S., Gish, W., Miller, W., Myers, E., & Lipman, D. (1990). "Basic local alignment search tool.". J. Mol. Biol. , 215:403-410. Amend, J., & Shock, E. (2001). Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiology Reviews , 25, 175-243. Aragno, M. (1978). Enrichment, isolation and preliminary characterization of a thermophilic, endospore-forming hydrogen bacterium. FEMS Micobiol. Lett. , 3: 13-15. Aragno, M. (1992). The Thermophilic, Aerobic, Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Trüper, M. Dworkin, W. Harder, & K. Schleifer, The Prokaryotes, a handbook on biology of bacteria. 2nd ed. vol. 4 (pp. 3917-3933.). New York: Springer Verlag. Aragno, M., & Schlegel, H. G. (1992). The mesophilic Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Truper, M. Dworkin, W. Harder, & K.-H. Schleifer, The Prokaryotes 2nd. ed. (pp. 344-384). New York: Springer. Ármannson, H. (2002, May 30-31). Erindi á ráðstefnu um málefni veitufyrirtækja . Grænt bókhald í jarðhita- samanburður á útblæstri við aðra orkugjafa . Akureyri, Iceland: Samorka. Bae, S., Kwak, K., Kim, S., Chung, S., & Igarashi, Y. (2001). Isolation and Characterization of CO2-Fixing Hydrogen -Oxidizing Marine 109 University of Akureyri Department of Natural Resource Science Bacteria. -

Characterization of the Uncommon Enzymes from (2004)
OPEN Mannosylglucosylglycerate biosynthesis SUBJECT AREAS: in the deep-branching phylum WATER MICROBIOLOGY MARINE MICROBIOLOGY Planctomycetes: characterization of the HOMEOSTASIS MULTIENZYME COMPLEXES uncommon enzymes from Rhodopirellula Received baltica 13 March 2013 Sofia Cunha1, Ana Filipa d’Avo´1, Ana Mingote2, Pedro Lamosa3, Milton S. da Costa1,4 & Joana Costa1,4 Accepted 23 July 2013 1Center for Neuroscience and Cell Biology, University of Coimbra, 3004-517 Coimbra, Portugal, 2Instituto de Tecnologia Quı´mica Published e Biolo´gica, Universidade Nova de Lisboa, 2780-157 Oeiras, Portugal, 3Centro de Ressonaˆncia Magne´tica Anto´nio Xavier, 7 August 2013 Instituto de Tecnologia Quı´mica e Biolo´gica, Universidade Nova de Lisboa, 2781-901 Oeiras, Portugal, 4Department of Life Sciences, University of Coimbra, Apartado 3046, 3001-401 Coimbra, Portugal. Correspondence and The biosynthetic pathway for the rare compatible solute mannosylglucosylglycerate (MGG) accumulated by requests for materials Rhodopirellula baltica, a marine member of the phylum Planctomycetes, has been elucidated. Like one of the should be addressed to pathways used in the thermophilic bacterium Petrotoga mobilis, it has genes coding for J.C. ([email protected].) glucosyl-3-phosphoglycerate synthase (GpgS) and mannosylglucosyl-3-phosphoglycerate (MGPG) synthase (MggA). However, unlike Ptg. mobilis, the mesophilic R. baltica uses a novel and very specific MGPG phosphatase (MggB). It also lacks a key enzyme of the alternative pathway in Ptg. mobilis – the mannosylglucosylglycerate synthase (MggS) that catalyses the condensation of glucosylglycerate with GDP-mannose to produce MGG. The R. baltica enzymes GpgS, MggA, and MggB were expressed in E. coli and characterized in terms of kinetic parameters, substrate specificity, temperature and pH dependence. -

Dominio Archaea
FILOGENIA DE LOS SERES VIVOS: DOMINIO ARCHAEA Nuria Garzón Pinto Facultad de Farmacia Universidad de Sevilla Septiembre de 2017 FILOGENIA DE LOS SERES VIVOS: DOMINIO ARCHAEA TRABAJO FIN DE GRADO Nuria Garzón Pinto Tutores: Antonio Ventosa Ucero y Cristina Sánchez-Porro Álvarez Tipología del trabajo: Revisión bibliográfica Grado en Farmacia. Facultad de Farmacia Departamento de Microbiología y Parasitología (Área de Microbiología) Universidad de Sevilla Sevilla, septiembre de 2017 RESUMEN A lo largo de la historia, la clasificación de los seres vivos ha ido variando en función de las diversas aportaciones científicas que se iban proponiendo, y la historia evolutiva de los organismos ha sido durante mucho tiempo algo que no se lograba conocer con claridad. Actualmente, gracias sobre todo a las ideas aportadas por Carl Woese y colaboradores, se sabe que los seres vivos se clasifican en 3 dominios (Bacteria, Eukarya y Archaea) y se conocen las herramientas que nos permiten realizar estudios filogenéticos, es decir, estudiar el origen de las especies. La herramienta principal, y en base a la cual se ha realizado la clasificación actual es el ARNr 16S. Sin embargo, hoy día sedispone de otros métodos que ayudan o complementan los análisis de la evolución de los seres vivos. En este trabajo se analiza cómo surgió el dominio Archaea, se describen las características y aspectos más importantes de las especies este grupo y se compara con el resto de dominios (Bacteria y Eukarya). Las arqueas han despertado un gran interés científico y han sido investigadas sobre todo por su capacidad para adaptarse y desarrollarse en ambientes extremos. -

Access to Electronic Thesis
Access to Electronic Thesis Author: Khalid Salim Al-Abri Thesis title: USE OF MOLECULAR APPROACHES TO STUDY THE OCCURRENCE OF EXTREMOPHILES AND EXTREMODURES IN NON-EXTREME ENVIRONMENTS Qualification: PhD This electronic thesis is protected by the Copyright, Designs and Patents Act 1988. No reproduction is permitted without consent of the author. It is also protected by the Creative Commons Licence allowing Attributions-Non-commercial-No derivatives. If this electronic thesis has been edited by the author it will be indicated as such on the title page and in the text. USE OF MOLECULAR APPROACHES TO STUDY THE OCCURRENCE OF EXTREMOPHILES AND EXTREMODURES IN NON-EXTREME ENVIRONMENTS By Khalid Salim Al-Abri Msc., University of Sultan Qaboos, Muscat, Oman Mphil, University of Sheffield, England Thesis submitted in partial fulfillment for the requirements of the Degree of Doctor of Philosophy in the Department of Molecular Biology and Biotechnology, University of Sheffield, England 2011 Introductory Pages I DEDICATION To the memory of my father, loving mother, wife “Muneera” and son “Anas”, brothers and sisters. Introductory Pages II ACKNOWLEDGEMENTS Above all, I thank Allah for helping me in completing this project. I wish to express my thanks to my supervisor Professor Milton Wainwright, for his guidance, supervision, support, understanding and help in this project. In addition, he also stood beside me in all difficulties that faced me during study. My thanks are due to Dr. D. J. Gilmour for his co-supervision, technical assistance, his time and understanding that made some of my laboratory work easier. In the Ministry of Regional Municipalities and Water Resources, I am particularly grateful to Engineer Said Al Alawi, Director General of Health Control, for allowing me to carry out my PhD study at the University of Sheffield.