Electron Donors and Acceptors for Members of the Family Beggiatoaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Delegate List
About CTBUH The Council on Tall Buildings and Urban Habitat (CTBUH) is the world’s leading resource for professionals focused on the inception, design, construction, and operation of tall buildings and future cities. Founded in 1969 and headquartered at Chicago’s historic Monroe Building, the CTBUH is a not-for-profit organization with an Asia Headquarters office at Tongji University, Shanghai; a Research Office at Iuav University, Venice, Italy; and an Academic Office at the Illinois Institute of Technology, Chicago. CTBUH facilitates the exchange of the latest knowledge available on tall buildings around the world through publications, research, events, working groups, web resources, and its extensive network of international representatives. The Council’s research department is spearheading the investigation of the next generation of tall buildings by aiding original Dubai & Abu Dhabi, UAE | 20–25 October research on sustainability and key development issues. The Council’s free database on tall buildings, The Skyscraper Center, is updated daily with detailed information, images, data, and news. The CTBUH also developed the international standards for measuring tall building height and is recognized as the arbiter for bestowing such designations as “The World’s Tallest Building.” Delegate List www.ctbuh.org | www.skyscrapercenter.com ctbuh2018.org #CTBUH2018 CTBUH2018_DelegateList_Cover.indd 2-3 10/12/2018 4:40:55 PM Many Thanks to All of Our Sponsors Delegate List: What’s Inside? Diamond Attendance Analysis 3 Top Regions & Companies Represented Delegate List by Company 6 Listed Alphabetically by Affi liation Platinum Delegate List by Surname 26 Listed Alphabetically by Surname Gold 1300+ DELEGATES 278 PRESENTERS 27 OFF-SITE WME consultants PROGRAMS 8 TRACKS Silver 4 1 EVENINGS OF GREAT RECEPTIONS SYMPOSIUMS 3 CONFERENCE! PROGRAM ROOMS 5 SPONSORS 68 128 CITIES 447 COMPANIES Bronze Supported By: COUNTRIES 54 2 Representation by Region Note: This registration list includes the 1240 delegates that were registered by Monday 8 October. -

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

Regeneration of Unconventional Natural Gas by Methanogens Co
www.nature.com/scientificreports OPEN Regeneration of unconventional natural gas by methanogens co‑existing with sulfate‑reducing prokaryotes in deep shale wells in China Yimeng Zhang1,2,3, Zhisheng Yu1*, Yiming Zhang4 & Hongxun Zhang1 Biogenic methane in shallow shale reservoirs has been proven to contribute to economic recovery of unconventional natural gas. However, whether the microbes inhabiting the deeper shale reservoirs at an average depth of 4.1 km and even co-occurring with sulfate-reducing prokaryote (SRP) have the potential to produce biomethane is still unclear. Stable isotopic technique with culture‑dependent and independent approaches were employed to investigate the microbial and functional diversity related to methanogenic pathways and explore the relationship between SRP and methanogens in the shales in the Sichuan Basin, China. Although stable isotopic ratios of the gas implied a thermogenic origin for methane, the decreased trend of stable carbon and hydrogen isotope value provided clues for increasing microbial activities along with sustained gas production in these wells. These deep shale-gas wells harbored high abundance of methanogens (17.2%) with ability of utilizing various substrates for methanogenesis, which co-existed with SRP (6.7%). All genes required for performing methylotrophic, hydrogenotrophic and acetoclastic methanogenesis were present. Methane production experiments of produced water, with and without additional available substrates for methanogens, further confrmed biomethane production via all three methanogenic pathways. Statistical analysis and incubation tests revealed the partnership between SRP and methanogens under in situ sulfate concentration (~ 9 mg/L). These results suggest that biomethane could be produced with more fexible stimulation strategies for unconventional natural gas recovery even at the higher depths and at the presence of SRP. -

Ancient Metaproteomics: a Novel Approach for Understanding Disease And
Ancient metaproteomics: a novel approach for understanding disease and diet in the archaeological record Jessica Hendy PhD University of York Archaeology August, 2015 ii Abstract Proteomics is increasingly being applied to archaeological samples following technological developments in mass spectrometry. This thesis explores how these developments may contribute to the characterisation of disease and diet in the archaeological record. This thesis has a three-fold aim; a) to evaluate the potential of shotgun proteomics as a method for characterising ancient disease, b) to develop the metaproteomic analysis of dental calculus as a tool for understanding both ancient oral health and patterns of individual food consumption and c) to apply these methodological developments to understanding individual lifeways of people enslaved during the 19th century transatlantic slave trade. This thesis demonstrates that ancient metaproteomics can be a powerful tool for identifying microorganisms in the archaeological record, characterising the functional profile of ancient proteomes and accessing individual patterns of food consumption with high taxonomic specificity. In particular, analysis of dental calculus may be an extremely valuable tool for understanding the aetiology of past oral diseases. Results of this study highlight the value of revisiting previous studies with more recent methodological approaches and demonstrate that biomolecular preservation can have a significant impact on the effectiveness of ancient proteins as an archaeological tool for this characterisation. Using the approaches developed in this study we have the opportunity to increase the visibility of past diseases and their aetiology, as well as develop a richer understanding of individual lifeways through the production of molecular life histories. iii iv List of Contents Abstract ............................................................................................................................... -
A Deep Learning Approach to Pattern Recognition for Short DNA Sequences”
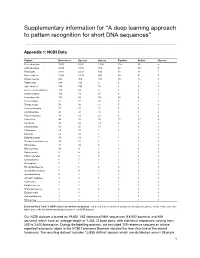
Supplementary information for “A deep learning approach to pattern recognition for short DNA sequences” Appendix 1: NCBI Data Phylum References Species Genera Families Orders Classes Proteobacteria 7,053 5,061 1,106 158 55 9 Actinobacteria 4,768 3,313 383 68 29 6 Firmicutes 3,814 2,531 499 56 13 7 Bacteroidetes 1,934 1,525 360 39 8 7 Euryarchaeota 834 450 100 28 13 8 Tenericutes 266 195 8 5 4 1 Spirochaetes 146 100 16 6 4 1 Deinococcus-Thermus 118 99 9 3 2 1 Crenarchaeota 113 61 27 8 5 1 Cyanobacteria 113 88 59 30 8 2 Fusobacteria 75 37 10 2 1 1 Thermotogae 70 48 13 5 4 1 Verrucomicrobia 57 53 22 7 4 3 Acidobacteria 45 41 19 5 5 4 Planctomycetes 44 31 23 5 3 2 Chloroflexi 44 35 26 15 12 8 Aquificae 43 32 14 4 2 1 Synergistetes 31 25 15 1 1 1 Chlamydiae 28 18 7 5 2 1 Chlorobi 21 16 5 1 1 1 Deferribacteres 15 11 7 1 1 1 Thermodesulfobacteria 14 12 5 1 1 1 Nitrospirae 11 10 3 1 1 1 Fibrobacteres 10 4 3 3 3 3 Balneolaeota 9 9 4 1 1 1 Chrysiogenetes 6 4 3 1 1 1 Lentisphaerae 6 5 3 3 3 2 Dictyoglomi 5 2 1 1 1 1 Rhodothermaeota 5 5 3 2 1 1 Gemmatimonadetes 5 4 3 2 2 2 Ignavibacteriae 4 2 2 2 1 1 Armatimonadetes 4 3 3 3 3 3 Caldiserica 3 1 1 1 1 1 Calditrichaeota 2 2 1 1 1 1 Thaumarchaeota 2 2 2 2 2 2 Elusimicrobia 2 1 1 1 1 1 Kiritimatiellaeota 1 1 1 1 1 1 Nitrospinae 1 1 1 1 1 1 Extended Data Table 1: NCBI dataset breakdown by phylum. -

Uncovering REDD+ Readiness in Mexico: Actors, Discourses and Benefit-Sharing
Uncovering REDD+ readiness in Mexico: Actors, discourses and benefit-sharing PhD Thesis Jovanka Špirić Under the supervision of: Dr. Esteve Corbera (UAB) Dr. Victoria Reyes-García (ICREA-UAB) Dr. Luciana Porter-Bolland (INECOL) PhD Programme in Environmental Science and Technology Institut de Ciència i Tecnologia Ambientals, ICTA Universitat Autònoma de Barcelona, UAB December 2015 Mojoj porodici Abstract Reducing Emissions from Deforestation and forest Degradation, plus conservation, sustainable management of forests and enhancement of forest carbon stocks (REDD+) is an international policy mechanism that seeks to mitigate climate change, while potentially alleviating poverty and contributing towards biodiversity conservation in developing countries. The United Nations Framework Convention on Climate Change (UNFCCC) laid the foundations for REDD+ design and implementation in 2005 and the mechanism’s architecture was finalised in 2015. During that period, parties to the UNFCCC debated and developed procedures and guidelines on REDD+ technical and governance issues, including for example how to guarantee the meaningful participation of all relevant stakeholders and how to respect the rights of indigenous peoples and local communities. In parallel, several developing countries, supported by multilateral and bilateral aid, entered the so-called REDD+ readiness phase and started developing national strategies for implementing REDD+ activities through specific policies and actions. This thesis addresses three main issues of concern for REDD+ scholars and practitioners using Mexico’s readiness process as an example. First, it analyses the design and legitimacy of the institutional arrangements established by the Mexican government to draft the REDD+ national strategy. Second, it identifies the REDD+ discourses mobilised by the actors involved in the country’s REDD+ readiness process and it highlights how such discourses are reflected in national policy documents, thus shedding light on dominant ideas and narratives permeating into the national strategy. -

Differentiating Two Closely Related Alexandrium Species Using Comparative Quantitative Proteomics
toxins Article Differentiating Two Closely Related Alexandrium Species Using Comparative Quantitative Proteomics Bryan John J. Subong 1,2,* , Arturo O. Lluisma 1, Rhodora V. Azanza 1 and Lilibeth A. Salvador-Reyes 1,* 1 Marine Science Institute, University of the Philippines- Diliman, Velasquez Street, Quezon City 1101, Philippines; [email protected] (A.O.L.); [email protected] (R.V.A.) 2 Department of Chemistry, The University of Tokyo, 7-3-1 Hongo, Bunkyo City, Tokyo 113-8654, Japan * Correspondence: [email protected] (B.J.J.S.); [email protected] (L.A.S.-R.) Abstract: Alexandrium minutum and Alexandrium tamutum are two closely related harmful algal bloom (HAB)-causing species with different toxicity. Using isobaric tags for relative and absolute quantita- tion (iTRAQ)-based quantitative proteomics and two-dimensional differential gel electrophoresis (2D-DIGE), a comprehensive characterization of the proteomes of A. minutum and A. tamutum was performed to identify the cellular and molecular underpinnings for the dissimilarity between these two species. A total of 1436 proteins and 420 protein spots were identified using iTRAQ-based proteomics and 2D-DIGE, respectively. Both methods revealed little difference (10–12%) between the proteomes of A. minutum and A. tamutum, highlighting that these organisms follow similar cellular and biological processes at the exponential stage. Toxin biosynthetic enzymes were present in both organisms. However, the gonyautoxin-producing A. minutum showed higher levels of osmotic growth proteins, Zn-dependent alcohol dehydrogenase and type-I polyketide synthase compared to the non-toxic A. tamutum. Further, A. tamutum had increased S-adenosylmethionine transferase that may potentially have a negative feedback mechanism to toxin biosynthesis. -

The Microbial Ecology of Sulfur Transformations in Oyster Pond, Woods Hole, Massachusetts
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 1970 THE MICROBIAL ECOLOGY OF SULFUR TRANSFORMATIONS IN OYSTER POND, WOODS HOLE, MASSACHUSETTS FRANK W. BARVENIK Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation BARVENIK, FRANK W., "THE MICROBIAL ECOLOGY OF SULFUR TRANSFORMATIONS IN OYSTER POND, WOODS HOLE, MASSACHUSETTS" (1970). Doctoral Dissertations. 933. https://scholars.unh.edu/dissertation/933 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. 71-5863 BARVENIK, Frank W., 1943- | THE MICROBIAL ECOLOGY OF SULFUR TRANSFORMATIONS I IN OYSTER POND, WOODS HOLE, MASSACHUSETTS. f i University of New Hampshire, Ph.D., 1970 f Microbiology University Microfilms, Inc., Ann Arbor, Michigan THIS DISSERTATION HAS BEEN MICROFILMED EXACTLY AS RECEIVED THE MICROBIAL ECOLOGY OF SULFUR TRANSFORMATIONS IN OYSTER POND, WOODS HOLE, MASSACHUSETTS by FRANK W. BARVENIK B.A., University of Connecticut, 1965 A THESIS Submitted to the University of New Hampshire In Partial Fulfillment of The Requirements for the Degree of Doctor of Philosophy Graduate School Department of Microbiology June, 1970 This thesis has been examined and approved. C. __ _ Thesisis director, #£len2alen E. Jones, Prof. of Microbiology William R. Chesbro, Prof. of Microbiology ______ jawrence W. Slanetz, Prof. of M igroipiology IjHtVX 7 ~ _____ ___ _________________ Henri E. Gaudettfy, Asso. Prcff. of Geiology Sanuiel C. -

Novel Bacterial Diversity in an Anchialine Blue Hole On
NOVEL BACTERIAL DIVERSITY IN AN ANCHIALINE BLUE HOLE ON ABACO ISLAND, BAHAMAS A Thesis by BRETT CHRISTOPHER GONZALEZ Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December 2010 Major Subject: Wildlife and Fisheries Sciences NOVEL BACTERIAL DIVERSITY IN AN ANCHIALINE BLUE HOLE ON ABACO ISLAND, BAHAMAS A Thesis by BRETT CHRISTOPHER GONZALEZ Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Thomas Iliffe Committee Members, Robin Brinkmeyer Daniel Thornton Head of Department, Thomas Lacher, Jr. December 2010 Major Subject: Wildlife and Fisheries Sciences iii ABSTRACT Novel Bacterial Diversity in an Anchialine Blue Hole on Abaco Island, Bahamas. (December 2010) Brett Christopher Gonzalez, B.S., Texas A&M University at Galveston Chair of Advisory Committee: Dr. Thomas Iliffe Anchialine blue holes found in the interior of the Bahama Islands have distinct fresh and salt water layers, with vertical mixing, and dysoxic to anoxic conditions below the halocline. Scientific cave diving exploration and microbiological investigations of Cherokee Road Extension Blue Hole on Abaco Island have provided detailed information about the water chemistry of the vertically stratified water column. Hydrologic parameters measured suggest that circulation of seawater is occurring deep within the platform. Dense microbial assemblages which occurred as mats on the cave walls below the halocline were investigated through construction of 16S rRNA clone libraries, finding representatives across several bacterial lineages including Chlorobium and OP8. -

Article-Associated Bac- Teria and Colony Isolation in Soft Agar Medium for Bacteria Unable to Grow at the Air-Water Interface
Biogeosciences, 8, 1955–1970, 2011 www.biogeosciences.net/8/1955/2011/ Biogeosciences doi:10.5194/bg-8-1955-2011 © Author(s) 2011. CC Attribution 3.0 License. Diversity of cultivated and metabolically active aerobic anoxygenic phototrophic bacteria along an oligotrophic gradient in the Mediterranean Sea C. Jeanthon1,2, D. Boeuf1,2, O. Dahan1,2, F. Le Gall1,2, L. Garczarek1,2, E. M. Bendif1,2, and A.-C. Lehours3 1Observatoire Oceanologique´ de Roscoff, UMR7144, INSU-CNRS – Groupe Plancton Oceanique,´ 29680 Roscoff, France 2UPMC Univ Paris 06, UMR7144, Adaptation et Diversite´ en Milieu Marin, Station Biologique de Roscoff, 29680 Roscoff, France 3CNRS, UMR6023, Microorganismes: Genome´ et Environnement, Universite´ Blaise Pascal, 63177 Aubiere` Cedex, France Received: 21 April 2011 – Published in Biogeosciences Discuss.: 5 May 2011 Revised: 7 July 2011 – Accepted: 8 July 2011 – Published: 20 July 2011 Abstract. Aerobic anoxygenic phototrophic (AAP) bac- detected in the eastern basin, reflecting the highest diver- teria play significant roles in the bacterioplankton produc- sity of pufM transcripts observed in this ultra-oligotrophic tivity and biogeochemical cycles of the surface ocean. In region. To our knowledge, this is the first study to document this study, we applied both cultivation and mRNA-based extensively the diversity of AAP isolates and to unveil the ac- molecular methods to explore the diversity of AAP bacte- tive AAP community in an oligotrophic marine environment. ria along an oligotrophic gradient in the Mediterranean Sea By pointing out the discrepancies between culture-based and in early summer 2008. Colony-forming units obtained on molecular methods, this study highlights the existing gaps in three different agar media were screened for the production the understanding of the AAP bacteria ecology, especially in of bacteriochlorophyll-a (BChl-a), the light-harvesting pig- the Mediterranean Sea and likely globally. -
![Arxiv:2105.11503V2 [Physics.Bio-Ph] 26 May 2021 3.1 Geometry and Swimming Speeds of the Cells](https://docslib.b-cdn.net/cover/5911/arxiv-2105-11503v2-physics-bio-ph-26-may-2021-3-1-geometry-and-swimming-speeds-of-the-cells-465911.webp)
Arxiv:2105.11503V2 [Physics.Bio-Ph] 26 May 2021 3.1 Geometry and Swimming Speeds of the Cells
The Bank Of Swimming Organisms at the Micron Scale (BOSO-Micro) Marcos F. Velho Rodrigues1, Maciej Lisicki2, Eric Lauga1,* 1 Department of Applied Mathematics and Theoretical Physics, University of Cambridge, Cambridge CB3 0WA, United Kingdom. 2 Faculty of Physics, University of Warsaw, Warsaw, Poland. *Email: [email protected] Abstract Unicellular microscopic organisms living in aqueous environments outnumber all other creatures on Earth. A large proportion of them are able to self-propel in fluids with a vast diversity of swimming gaits and motility patterns. In this paper we present a biophysical survey of the available experimental data produced to date on the characteristics of motile behaviour in unicellular microswimmers. We assemble from the available literature empirical data on the motility of four broad categories of organisms: bacteria (and archaea), flagellated eukaryotes, spermatozoa and ciliates. Whenever possible, we gather the following biological, morphological, kinematic and dynamical parameters: species, geometry and size of the organisms, swimming speeds, actuation frequencies, actuation amplitudes, number of flagella and properties of the surrounding fluid. We then organise the data using the established fluid mechanics principles for propulsion at low Reynolds number. Specifically, we use theoretical biophysical models for the locomotion of cells within the same taxonomic groups of organisms as a means of rationalising the raw material we have assembled, while demonstrating the variability for organisms of different species within the same group. The material gathered in our work is an attempt to summarise the available experimental data in the field, providing a convenient and practical reference point for future studies. Contents 1 Introduction 2 2 Methods 4 2.1 Propulsion at low Reynolds number . -

7. References
University of Akureyri Department of Natural Resource Science 7. References Ammann, E. C., Reed, L. L., & Durichek, J. J. (1968). Gas consumptions and Growth Rate of Hydrogenomonas eutropha in Continuous Culture. Applied Microbiology , 16, (6), 822-826. Aguiar, P., Beveridge, T. J., & Reysenbach, A.-L. (2004). Sulfurihydrogenibium azorense, sp. nov., a thermophilic hydrogen oxidizing microaerophile from terrestrial hot springs in the Azores. International Journal of Systematic and Evolutionary Microbiology , 54, 33-39. Altschul, S., Gish, W., Miller, W., Myers, E., & Lipman, D. (1990). "Basic local alignment search tool.". J. Mol. Biol. , 215:403-410. Amend, J., & Shock, E. (2001). Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiology Reviews , 25, 175-243. Aragno, M. (1978). Enrichment, isolation and preliminary characterization of a thermophilic, endospore-forming hydrogen bacterium. FEMS Micobiol. Lett. , 3: 13-15. Aragno, M. (1992). The Thermophilic, Aerobic, Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Trüper, M. Dworkin, W. Harder, & K. Schleifer, The Prokaryotes, a handbook on biology of bacteria. 2nd ed. vol. 4 (pp. 3917-3933.). New York: Springer Verlag. Aragno, M., & Schlegel, H. G. (1992). The mesophilic Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Truper, M. Dworkin, W. Harder, & K.-H. Schleifer, The Prokaryotes 2nd. ed. (pp. 344-384). New York: Springer. Ármannson, H. (2002, May 30-31). Erindi á ráðstefnu um málefni veitufyrirtækja . Grænt bókhald í jarðhita- samanburður á útblæstri við aðra orkugjafa . Akureyri, Iceland: Samorka. Bae, S., Kwak, K., Kim, S., Chung, S., & Igarashi, Y. (2001). Isolation and Characterization of CO2-Fixing Hydrogen -Oxidizing Marine 109 University of Akureyri Department of Natural Resource Science Bacteria.