Distribution of Aerobic Arsenite Oxidase Genes Within the Aquificales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity of Understudied Archaeal and Bacterial Populations of Yellowstone National Park: from Genes to Genomes Daniel Colman
University of New Mexico UNM Digital Repository Biology ETDs Electronic Theses and Dissertations 7-1-2015 Diversity of understudied archaeal and bacterial populations of Yellowstone National Park: from genes to genomes Daniel Colman Follow this and additional works at: https://digitalrepository.unm.edu/biol_etds Recommended Citation Colman, Daniel. "Diversity of understudied archaeal and bacterial populations of Yellowstone National Park: from genes to genomes." (2015). https://digitalrepository.unm.edu/biol_etds/18 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in Biology ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. Daniel Robert Colman Candidate Biology Department This dissertation is approved, and it is acceptable in quality and form for publication: Approved by the Dissertation Committee: Cristina Takacs-Vesbach , Chairperson Robert Sinsabaugh Laura Crossey Diana Northup i Diversity of understudied archaeal and bacterial populations from Yellowstone National Park: from genes to genomes by Daniel Robert Colman B.S. Biology, University of New Mexico, 2009 DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Biology The University of New Mexico Albuquerque, New Mexico July 2015 ii DEDICATION I would like to dedicate this dissertation to my late grandfather, Kenneth Leo Colman, associate professor of Animal Science in the Wool laboratory at Montana State University, who even very near the end of his earthly tenure, thought it pertinent to quiz my knowledge of oxidized nitrogen compounds. He was a man of great curiosity about the natural world, and to whom I owe an acknowledgement for his legacy of intellectual (and actual) wanderlust. -
A Deep Learning Approach to Pattern Recognition for Short DNA Sequences”
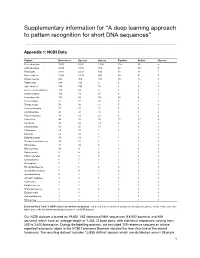
Supplementary information for “A deep learning approach to pattern recognition for short DNA sequences” Appendix 1: NCBI Data Phylum References Species Genera Families Orders Classes Proteobacteria 7,053 5,061 1,106 158 55 9 Actinobacteria 4,768 3,313 383 68 29 6 Firmicutes 3,814 2,531 499 56 13 7 Bacteroidetes 1,934 1,525 360 39 8 7 Euryarchaeota 834 450 100 28 13 8 Tenericutes 266 195 8 5 4 1 Spirochaetes 146 100 16 6 4 1 Deinococcus-Thermus 118 99 9 3 2 1 Crenarchaeota 113 61 27 8 5 1 Cyanobacteria 113 88 59 30 8 2 Fusobacteria 75 37 10 2 1 1 Thermotogae 70 48 13 5 4 1 Verrucomicrobia 57 53 22 7 4 3 Acidobacteria 45 41 19 5 5 4 Planctomycetes 44 31 23 5 3 2 Chloroflexi 44 35 26 15 12 8 Aquificae 43 32 14 4 2 1 Synergistetes 31 25 15 1 1 1 Chlamydiae 28 18 7 5 2 1 Chlorobi 21 16 5 1 1 1 Deferribacteres 15 11 7 1 1 1 Thermodesulfobacteria 14 12 5 1 1 1 Nitrospirae 11 10 3 1 1 1 Fibrobacteres 10 4 3 3 3 3 Balneolaeota 9 9 4 1 1 1 Chrysiogenetes 6 4 3 1 1 1 Lentisphaerae 6 5 3 3 3 2 Dictyoglomi 5 2 1 1 1 1 Rhodothermaeota 5 5 3 2 1 1 Gemmatimonadetes 5 4 3 2 2 2 Ignavibacteriae 4 2 2 2 1 1 Armatimonadetes 4 3 3 3 3 3 Caldiserica 3 1 1 1 1 1 Calditrichaeota 2 2 1 1 1 1 Thaumarchaeota 2 2 2 2 2 2 Elusimicrobia 2 1 1 1 1 1 Kiritimatiellaeota 1 1 1 1 1 1 Nitrospinae 1 1 1 1 1 1 Extended Data Table 1: NCBI dataset breakdown by phylum. -

Thermophilic Lithotrophy and Phototrophy in an Intertidal, Iron-Rich, Geothermal Spring 2 3 Lewis M
bioRxiv preprint doi: https://doi.org/10.1101/428698; this version posted September 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Thermophilic Lithotrophy and Phototrophy in an Intertidal, Iron-rich, Geothermal Spring 2 3 Lewis M. Ward1,2,3*, Airi Idei4, Mayuko Nakagawa2,5, Yuichiro Ueno2,5,6, Woodward W. 4 Fischer3, Shawn E. McGlynn2* 5 6 1. Department of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138 USA 7 2. Earth-Life Science Institute, Tokyo Institute of Technology, Meguro, Tokyo, 152-8550, Japan 8 3. Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 9 91125 USA 10 4. Department of Biological Sciences, Tokyo Metropolitan University, Hachioji, Tokyo 192-0397, 11 Japan 12 5. Department of Earth and Planetary Sciences, Tokyo Institute of Technology, Meguro, Tokyo, 13 152-8551, Japan 14 6. Department of Subsurface Geobiological Analysis and Research, Japan Agency for Marine-Earth 15 Science and Technology, Natsushima-cho, Yokosuka 237-0061, Japan 16 Correspondence: [email protected] or [email protected] 17 18 Abstract 19 Hydrothermal systems, including terrestrial hot springs, contain diverse and systematic 20 arrays of geochemical conditions that vary over short spatial scales due to progressive interaction 21 between the reducing hydrothermal fluids, the oxygenated atmosphere, and in some cases 22 seawater. At Jinata Onsen, on Shikinejima Island, Japan, an intertidal, anoxic, iron- and 23 hydrogen-rich hot spring mixes with the oxygenated atmosphere and sulfate-rich seawater over 24 short spatial scales, creating an enormous range of redox environments over a distance ~10 m. -

7. References
University of Akureyri Department of Natural Resource Science 7. References Ammann, E. C., Reed, L. L., & Durichek, J. J. (1968). Gas consumptions and Growth Rate of Hydrogenomonas eutropha in Continuous Culture. Applied Microbiology , 16, (6), 822-826. Aguiar, P., Beveridge, T. J., & Reysenbach, A.-L. (2004). Sulfurihydrogenibium azorense, sp. nov., a thermophilic hydrogen oxidizing microaerophile from terrestrial hot springs in the Azores. International Journal of Systematic and Evolutionary Microbiology , 54, 33-39. Altschul, S., Gish, W., Miller, W., Myers, E., & Lipman, D. (1990). "Basic local alignment search tool.". J. Mol. Biol. , 215:403-410. Amend, J., & Shock, E. (2001). Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiology Reviews , 25, 175-243. Aragno, M. (1978). Enrichment, isolation and preliminary characterization of a thermophilic, endospore-forming hydrogen bacterium. FEMS Micobiol. Lett. , 3: 13-15. Aragno, M. (1992). The Thermophilic, Aerobic, Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Trüper, M. Dworkin, W. Harder, & K. Schleifer, The Prokaryotes, a handbook on biology of bacteria. 2nd ed. vol. 4 (pp. 3917-3933.). New York: Springer Verlag. Aragno, M., & Schlegel, H. G. (1992). The mesophilic Hydrogen-Oxidizing (Knallgas) Bacteria. In A. Balows, H. Truper, M. Dworkin, W. Harder, & K.-H. Schleifer, The Prokaryotes 2nd. ed. (pp. 344-384). New York: Springer. Ármannson, H. (2002, May 30-31). Erindi á ráðstefnu um málefni veitufyrirtækja . Grænt bókhald í jarðhita- samanburður á útblæstri við aðra orkugjafa . Akureyri, Iceland: Samorka. Bae, S., Kwak, K., Kim, S., Chung, S., & Igarashi, Y. (2001). Isolation and Characterization of CO2-Fixing Hydrogen -Oxidizing Marine 109 University of Akureyri Department of Natural Resource Science Bacteria. -

Characterization of the Uncommon Enzymes from (2004)
OPEN Mannosylglucosylglycerate biosynthesis SUBJECT AREAS: in the deep-branching phylum WATER MICROBIOLOGY MARINE MICROBIOLOGY Planctomycetes: characterization of the HOMEOSTASIS MULTIENZYME COMPLEXES uncommon enzymes from Rhodopirellula Received baltica 13 March 2013 Sofia Cunha1, Ana Filipa d’Avo´1, Ana Mingote2, Pedro Lamosa3, Milton S. da Costa1,4 & Joana Costa1,4 Accepted 23 July 2013 1Center for Neuroscience and Cell Biology, University of Coimbra, 3004-517 Coimbra, Portugal, 2Instituto de Tecnologia Quı´mica Published e Biolo´gica, Universidade Nova de Lisboa, 2780-157 Oeiras, Portugal, 3Centro de Ressonaˆncia Magne´tica Anto´nio Xavier, 7 August 2013 Instituto de Tecnologia Quı´mica e Biolo´gica, Universidade Nova de Lisboa, 2781-901 Oeiras, Portugal, 4Department of Life Sciences, University of Coimbra, Apartado 3046, 3001-401 Coimbra, Portugal. Correspondence and The biosynthetic pathway for the rare compatible solute mannosylglucosylglycerate (MGG) accumulated by requests for materials Rhodopirellula baltica, a marine member of the phylum Planctomycetes, has been elucidated. Like one of the should be addressed to pathways used in the thermophilic bacterium Petrotoga mobilis, it has genes coding for J.C. ([email protected].) glucosyl-3-phosphoglycerate synthase (GpgS) and mannosylglucosyl-3-phosphoglycerate (MGPG) synthase (MggA). However, unlike Ptg. mobilis, the mesophilic R. baltica uses a novel and very specific MGPG phosphatase (MggB). It also lacks a key enzyme of the alternative pathway in Ptg. mobilis – the mannosylglucosylglycerate synthase (MggS) that catalyses the condensation of glucosylglycerate with GDP-mannose to produce MGG. The R. baltica enzymes GpgS, MggA, and MggB were expressed in E. coli and characterized in terms of kinetic parameters, substrate specificity, temperature and pH dependence. -

And Thermo-Adaptation in Hyperthermophilic Archaea: Identification of Compatible Solutes, Accumulation Profiles, and Biosynthetic Routes in Archaeoglobus Spp
Universidade Nova de Lisboa Osmo- andInstituto thermo de Tecnologia-adaptation Química e Biológica in hyperthermophilic Archaea: Subtitle Subtitle Luís Pedro Gafeira Gonçalves Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. OH OH OH CDP c c c - CMP O O - PPi O3P P CTP O O O OH OH OH OH OH OH O- C C C O P O O P i Dissertation presented to obtain the Ph.D degree in BiochemistryO O- Instituto de Tecnologia Química e Biológica | Universidade Nova de LisboaP OH O O OH OH OH Oeiras, Luís Pedro Gafeira Gonçalves January, 2008 2008 Universidade Nova de Lisboa Instituto de Tecnologia Química e Biológica Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. This dissertation was presented to obtain a Ph. D. degree in Biochemistry at the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa. By Luís Pedro Gafeira Gonçalves Supervised by Prof. Dr. Helena Santos Oeiras, January, 2008 Apoio financeiro da Fundação para a Ciência e Tecnologia (POCI 2010 – Formação Avançada para a Ciência – Medida IV.3) e FSE no âmbito do Quadro Comunitário de apoio, Bolsa de Doutoramento com a referência SFRH / BD / 5076 / 2001. ii ACKNOWNLEDGMENTS The work presented in this thesis, would not have been possible without the help, in terms of time and knowledge, of many people, to whom I am extremely grateful. Firstly and mostly, I need to thank my supervisor, Prof. Helena Santos, for her way of thinking science, her knowledge, her rigorous criticism, and her commitment to science. -

Molecular Evolution of the Oxygen-Binding Hemerythrin Domain
RESEARCH ARTICLE Molecular Evolution of the Oxygen-Binding Hemerythrin Domain Claudia Alvarez-Carreño1, Arturo Becerra1, Antonio Lazcano1,2* 1 Facultad de Ciencias, Universidad Nacional Autónoma de México, Apdo. Postal 70–407, Cd. Universitaria, 04510, Mexico City, Mexico, 2 Miembro de El Colegio Nacional, Ciudad de México, México * [email protected] a11111 Abstract Background The evolution of oxygenic photosynthesis during Precambrian times entailed the diversifica- tion of strategies minimizing reactive oxygen species-associated damage. Four families of OPEN ACCESS oxygen-carrier proteins (hemoglobin, hemerythrin and the two non-homologous families of Citation: Alvarez-Carreño C, Becerra A, Lazcano A arthropodan and molluscan hemocyanins) are known to have evolved independently the (2016) Molecular Evolution of the Oxygen-Binding Hemerythrin Domain. PLoS ONE 11(6): e0157904. capacity to bind oxygen reversibly, providing cells with strategies to cope with the evolution- doi:10.1371/journal.pone.0157904 ary pressure of oxygen accumulation. Oxygen-binding hemerythrin was first studied in Editor: Nikolas Nikolaidis, California State University marine invertebrates but further research has made it clear that it is present in the three Fullerton, UNITED STATES domains of life, strongly suggesting that its origin predated the emergence of eukaryotes. Received: April 5, 2016 Accepted: June 7, 2016 Results Published: June 23, 2016 Oxygen-binding hemerythrins are a monophyletic sub-group of the hemerythrin/HHE (histi- dine, histidine, glutamic acid) cation-binding domain. Oxygen-binding hemerythrin homo- Copyright: © 2016 Alvarez-Carreño et al. This is an open access article distributed under the terms of the logs were unambiguously identified in 367/2236 bacterial, 21/150 archaeal and 4/135 Creative Commons Attribution License, which permits eukaryotic genomes. -

NOC04013 Application.Pdf(PDF, 453
ER-AN-02N 10/02 Application for approval to import into FORM 2N containment any new organism that is not genetically modified, under Section 40 of the Page 1 Hazardous Substances and New Organisms Act 1996 FORM NO2N Application for approval to IMPORT INTO CONTAINMENT ANY NEW ORGANISM THAT IS NOT GENETICALLY MODIFIED under section 40 of the Hazardous Substances and New Organisms Act 1996 Application Title: Importation of sediment/water samples collected from hydrothermal seamounts outside New Zealand Territorial waters containing Extremophilic microorganisms Applicant Organisation: Institute of Geological & Nuclear Sciences ERMA Office use only Application Code: Formally received:____/____/____ ERMA NZ Contact: Initial Fee Paid: $ Application Status: ER-AN-02N 10/02 Application for approval to import into FORM 2N containment any new organism that is not genetically modified, under Section 40 of the Page 2 Hazardous Substances and New Organisms Act 1996 IMPORTANT 1. An associated User Guide is available for this form. You should read the User Guide before completing this form. If you need further guidance in completing this form please contact ERMA New Zealand. 2. This application form covers importation into containment of any new organism that is not genetically modified, under section 40 of the Act. 3. If you are making an application to import into containment a genetically modified organism you should complete Form NO2G, instead of this form (Form NO2N). 4. This form, together with form NO2G, replaces all previous versions of Form 2. Older versions should not now be used. You should periodically check with ERMA New Zealand or on the ERMA New Zealand web site for new versions of this form. -

BEING Aquifex Aeolicus: UNTANGLING a HYPERTHERMOPHILE‘S CHECKERED PAST
BEING Aquifex aeolicus: UNTANGLING A HYPERTHERMOPHILE‘S CHECKERED PAST by Robert J.M. Eveleigh Submitted in partial fulfillment of the requirements for the degree of Master of Science at Dalhousie University Halifax, Nova Scotia December 2011 © Copyright by Robert J.M. Eveleigh, 2011 DALHOUSIE UNIVERSITY DEPARTMENT OF COMPUTATIONAL BIOLOGY AND BIOINFORMATICS The undersigned hereby certify that they have read and recommend to the Faculty of Graduate Studies for acceptance a thesis entitled ―BEING Aquifex aeolicus: UNTANGLING A HYPERTHERMOPHILE‘S CHECKERED PAST‖ by Robert J.M. Eveleigh in partial fulfillment of the requirements for the degree of Master of Science. Dated: December 13, 2011 Co-Supervisors: _________________________________ _________________________________ Readers: _________________________________ ii DALHOUSIE UNIVERSITY DATE: December 13, 2011 AUTHOR: Robert J.M. Eveleigh TITLE: BEING Aquifex aeolicus: UNTANGLING A HYPERTHERMOPHILE‘S CHECKERED PAST DEPARTMENT OR SCHOOL: Department of Computational Biology and Bioinformatics DEGREE: MSc CONVOCATION: May YEAR: 2012 Permission is herewith granted to Dalhousie University to circulate and to have copied for non-commercial purposes, at its discretion, the above title upon the request of individuals or institutions. I understand that my thesis will be electronically available to the public. The author reserves other publication rights, and neither the thesis nor extensive extracts from it may be printed or otherwise reproduced without the author‘s written permission. The author attests that permission has been obtained for the use of any copyrighted material appearing in the thesis (other than the brief excerpts requiring only proper acknowledgement in scholarly writing), and that all such use is clearly acknowledged. _______________________________ Signature of Author iii TABLE OF CONTENTS List of Tables .................................................................................................................... -

Lewis M. Ward *, Airi Idei , Mayuko Nakagawa , Yuichiro
bioRxiv preprint doi: https://doi.org/10.1101/428698; this version posted January 27, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Geochemical and metagenomic characterization of Jinata Onsen, a Proterozoic-analog hot spring, 2 reveals novel microbial diversity including iron-tolerant phototrophs and thermophilic lithotrophs 3 4 Lewis M. Ward1,2,3*, Airi Idei4, Mayuko Nakagawa2,5, Yuichiro Ueno2,5,6, Woodward W. 5 Fischer3, Shawn E. McGlynn2* 6 7 1. Department of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138 USA 8 2. Earth-Life Science Institute, Tokyo Institute of Technology, Meguro, Tokyo, 152-8550, Japan 9 3. Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 10 91125 USA 11 4. Department of Biological Sciences, Tokyo Metropolitan University, Hachioji, Tokyo 192-0397, 12 Japan 13 5. Department of Earth and Planetary Sciences, Tokyo Institute of Technology, Meguro, Tokyo, 14 152-8551, Japan 15 6. Department of Subsurface Geobiological Analysis and Research, Japan Agency for Marine-Earth 16 Science and Technology, Natsushima-cho, Yokosuka 237-0061, Japan 17 Correspondence: [email protected] or [email protected] 18 19 Abstract 20 Hydrothermal systems, including terrestrial hot springs, contain diverse geochemical 21 conditions that vary over short spatial scales due to progressive interaction between the reducing 22 hydrothermal fluids, the oxygenated atmosphere, and in some cases seawater. At Jinata Onsen, 23 on Shikinejima Island, Japan, an intertidal, anoxic, iron-rich hot spring mixes with the 24 oxygenated atmosphere and seawater over short spatial scales, creating a diversity of chemical 25 potentials and redox pairs over a distance ~10 m. -

Marinitoga Lauensis Sp. Nov., a Novel Deep-Sea Hydrothermal Vent Thermophilic Anaerobic Heterotroph with a Prophage
Portland State University PDXScholar Biology Faculty Publications and Presentations Biology 5-1-2019 Marinitoga Lauensis sp. nov., A Novel Deep-sea Hydrothermal Vent Thermophilic Anaerobic Heterotroph with a Prophage Stéphane L'Haridon Univ Brest, CNRS, IFREMER Léna Gouhie CNRS, IFREMER Emily St. John Portland State University, [email protected] Anna-Louise Reysenbach Portland State University, [email protected] Follow this and additional works at: https://pdxscholar.library.pdx.edu/bio_fac Let us know how access to this document benefits ou.y Citation Details Published as: L’Haridon, S., Gouhier, L., John, E. S., & Reysenbach, A. L. (2019). Marinitoga lauensis sp. nov., a novel deep-sea hydrothermal vent thermophilic anaerobic heterotroph with a prophage. Systematic and applied microbiology, 42(3), 343-347. This Post-Print is brought to you for free and open access. It has been accepted for inclusion in Biology Faculty Publications and Presentations by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Accepted Manuscript Title: Marinitoga lauensis sp. nov., a novel deep-sea hydrothermal vent thermophilic anaerobic heterotroph with a prophage Authors: Stephane´ L’Haridon, Lena´ Gouhier, Emily St. John, Anna-Louise Reysenbach PII: S0723-2020(18)30493-4 DOI: https://doi.org/10.1016/j.syapm.2019.02.006 Reference: SYAPM 25981 To appear in: Received date: 30 November 2018 Revised date: 17 February 2019 Accepted date: 26 February 2019 Please cite this article as: Stephane´ L’Haridon, Lena´ Gouhier, Emily St.John, Anna- Louise Reysenbach, Marinitoga lauensis sp.nov., a novel deep-sea hydrothermal vent thermophilic anaerobic heterotroph with a prophage, Systematic and Applied Microbiology https://doi.org/10.1016/j.syapm.2019.02.006 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Metabolic Potential and Survival Strategies of Microbial Communities
Metabolic potential and survival strategies of microbial communities across extreme temperature gradients on Deception Island volcano, Antarctica AMANDA BENDIA1, Leandro Nascimento Lemos2, Lucas Mendes1, Camila Signori1, Brendan Bohannan3, and Vivian Pellizari1 1University of Sao Paulo 2USP 3University of Oregon August 11, 2020 Abstract Active volcanoes in Antarctica, in contrast to the rest of the icy landscape, have remarkable temperature and geochemical gradients that could select for a wide variety of microbial adaptive mechanisms and metabolic pathways. Deception Island is a stratovolcano flooded by the sea, resulting in contrasting ecosystems such as permanent glaciers (<0 oC) and active fumaroles (up to 100 oC). Steep gradients in temperature, salinity and geochemistry over very short distances have been reported for Deception Island, and have been shown to effect microbial community structure and diversity. However, little is known regarding how these gradients affect ecosystem functioning, for example due to inhibition of key metabolic enzymes or pathways. In this study, we used shotgun metagenomics and metagenome-assembled genomes to explore how microbial functional diversity is shaped by extreme geochemical, salinity and temperature gradients in fumarole and glacier sediments. We observed that microbial communities from a 98 oC fumarole harbor specific hyperthermophilic molecular strategies, as well as reductive and autotrophic pathways, while those from <80 oC fumaroles possess more diverse metabolic and survival strategies capable of responding to fluctuating redox and temperature conditions. In contrast, glacier communities showed less diverse metabolic potentials, comprising mainly heterotrophic and carbon pathways. Through the reconstruction of genomes, we were able to clarify putative novel lifestyles of underrepresented taxonomic groups, especially those related to Nanoarchaeota and thermophilic ammonia-oxidizing archaeal lineages.