Effects of Hunting with Hounds on a Non-Target Species Living on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Population, Distribution and Conservation Status of Sitatunga (Tragelaphus Spekei) (Sclater) in Selected Wetlands in Uganda
POPULATION, DISTRIBUTION AND CONSERVATION STATUS OF SITATUNGA (TRAGELAPHUS SPEKEI) (SCLATER) IN SELECTED WETLANDS IN UGANDA Biological -Life history Biological -Ecologicl… Protection -Regulation of… 5 Biological -Dispersal Protection -Effectiveness… 4 Biological -Human tolerance Protection -proportion… 3 Status -National Distribtuion Incentive - habitat… 2 Status -National Abundance Incentive - species… 1 Status -National… Incentive - Effect of harvest 0 Status -National… Monitoring - confidence in… Status -National Major… Monitoring - methods used… Harvest Management -… Control -Confidence in… Harvest Management -… Control - Open access… Harvest Management -… Control of Harvest-in… Harvest Management -Aim… Control of Harvest-in… Harvest Management -… Control of Harvest-in… Tragelaphus spekii (sitatunga) NonSubmitted Detrimental to Findings (NDF) Research and Monitoring Unit Uganda Wildlife Authority (UWA) Plot 7 Kira Road Kamwokya, P.O. Box 3530 Kampala Uganda Email/Web - [email protected]/ www.ugandawildlife.org Prepared By Dr. Edward Andama (PhD) Lead consultant Busitema University, P. O. Box 236, Tororo Uganda Telephone: 0772464279 or 0704281806 E-mail: [email protected] [email protected], [email protected] Final Report i January 2019 Contents ACRONYMS, ABBREVIATIONS, AND GLOSSARY .......................................................... vii EXECUTIVE SUMMARY ....................................................................................................... viii 1.1Background ........................................................................................................................... -

Histories of Value Following Deer Populations Through the English Landscape from 1800 to the Present Day
Holly Marriott Webb Histories of Value Following Deer Populations Through the English Landscape from 1800 to the Present Day Master’s thesis in Global Environmental History 1 Abstract Marriott Webb, H. 2019. Histories of Value: Following Deer Populations Through the English Landscape from 1800 to the Present Day. Uppsala, Department of Archaeology and Ancient His- tory. Imagining the English landscape as an assemblage entangling deer and people throughout history, this thesis explores how changes in deer population connect to the ways deer have been valued from 1800 to the present day. Its methods are mixed, its sources are conversations – human voices in the ongoing historical negotiations of the multispecies body politic, the moot of people, animals, plants and things which shapes and orders the landscape assemblage. These conversations include interviews with people whose lives revolve around deer, correspondence with the organisations that hold sway over deer lives, analysis of modern media discourse around deer issues and exchanges with the history books. It finds that a non-linear increase in deer population over the time period has been accompanied by multiple changes in the way deer are valued as part of the English landscape. Ending with a reflection on how this history of value fits in to wider debates about the proper representation of animals, the nature of non-human agency, and trajectories of the Anthropocene, this thesis seeks to open up new ways of exploring questions about human- animal relationships in environmental history. Keywords: Assemblages, Deer, Deer population, England, Hunting, Landscape, Making killable, Moots, Multispecies, Nativist paradigm, Olwig, Pests, Place, Trash Animals, Tsing, United Kingdom, Wildlife management. -

Deer Legislation
Introduction This guide describes the general principles of the law relating to wild deer, it is not a full description of It is therefore advisable to carry written permission that law. It is important to study the full legislation as proof of your right to be on the land. to which this guide relates (see Further Information) Practitioners need to be fully conversant with current Exemptions. An offence is not committed if the legislation in order to make informed management perpetrator did so in the belief that he would have decisions and be sure that their actions are legal. been given consent if the owner or occupier knew of his doing it and the circumstances, or he has other The following defi nitions apply: lawful authority. “Deer” means deer of any species and includes the Ownership of deer. Deer which can roam carcass or any part thereof freely are wild animals and are not owned by, or “Night” means the period between 1 hour after the responsibility of, anyone. A wild deer becomes sunset and 1 hour before sunrise the property of the landowner when “reduced into “Vehicle” includes any vehicle including aircraft, possession” i.e. killed or captured, thus a culled deer hovercraft or boat is the property of the owner of the land on which it dies, a deer killed in a road accident is the property The law specifi cally relating to deer in England and of the owner of the highway, verge or land on which Wales is contained in the Deer Act 1991(Deer Act) it falls. -

The Effects of Upland Management Practices on Avian Diversity
The Effects of Upland Management Practices on Avian diversity Bronwen Daniel September 2010 A Thesis submitted in partial fulfilment of the requirements for the degree of Master of Science and the Diploma of Imperial College London 1 Contents 1. Introduction ................................................................................................................................. 3 2. Background................................................................................................................................. 11 2.1 Birds as indicators ................................................................................................................ 11 2.1.1 Upland birds ...................................................................................................................... 11 2.2 Management Practices......................................................................................................... 13 2.2.1 Grouse Moor Management........................................................................................... 15 2.2.2 Predator control ............................................................................................................ 16 2.2.3 Burning .......................................................................................................................... 17 2.2.4 Grazing Pressure............................................................................................................ 17 2.2.5 Implications of upland management for bird populations .......................................... -

A Scoping Review of Viral Diseases in African Ungulates
veterinary sciences Review A Scoping Review of Viral Diseases in African Ungulates Hendrik Swanepoel 1,2, Jan Crafford 1 and Melvyn Quan 1,* 1 Vectors and Vector-Borne Diseases Research Programme, Department of Veterinary Tropical Disease, Faculty of Veterinary Science, University of Pretoria, Pretoria 0110, South Africa; [email protected] (H.S.); [email protected] (J.C.) 2 Department of Biomedical Sciences, Institute of Tropical Medicine, 2000 Antwerp, Belgium * Correspondence: [email protected]; Tel.: +27-12-529-8142 Abstract: (1) Background: Viral diseases are important as they can cause significant clinical disease in both wild and domestic animals, as well as in humans. They also make up a large proportion of emerging infectious diseases. (2) Methods: A scoping review of peer-reviewed publications was performed and based on the guidelines set out in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews. (3) Results: The final set of publications consisted of 145 publications. Thirty-two viruses were identified in the publications and 50 African ungulates were reported/diagnosed with viral infections. Eighteen countries had viruses diagnosed in wild ungulates reported in the literature. (4) Conclusions: A comprehensive review identified several areas where little information was available and recommendations were made. It is recommended that governments and research institutions offer more funding to investigate and report viral diseases of greater clinical and zoonotic significance. A further recommendation is for appropriate One Health approaches to be adopted for investigating, controlling, managing and preventing diseases. Diseases which may threaten the conservation of certain wildlife species also require focused attention. -

Mammal Species Richness at a Catena and Nearby Waterholes During a Drought, Kruger National Park, South Africa
diversity Article Mammal Species Richness at a Catena and Nearby Waterholes during a Drought, Kruger National Park, South Africa Beanélri B. Janecke Animal, Wildlife & Grassland Sciences, University of the Free State, 205 Nelson Mandela Road, Park West, Bloemfontein 9301, South Africa; [email protected]; Tel.: +27-51-401-9030 Abstract: Catenas are undulating hillslopes on a granite geology characterised by different soil types that create an environmental gradient from crest to bottom. The main aim was to determine mammal species (>mongoose) present on one catenal slope and its waterholes and group them by feeding guild and body size. Species richness was highest at waterholes (21 species), followed by midslope (19) and sodic patch (16) on the catena. Small differences observed in species presence between zones and waterholes and between survey periods were not significant (p = 0.5267 and p = 0.9139). In total, 33 species were observed with camera traps: 18 herbivore species, 10 carnivores, two insectivores and three omnivores. Eight small mammal species, two dwarf antelopes, 11 medium, six large and six mega-sized mammals were observed. Some species might not have been recorded because of drought, seasonal movement or because they travelled outside the view of cameras. Mammal presence is determined by food availability and accessibility, space, competition, distance to water, habitat preferences, predators, body size, social behaviour, bound to territories, etc. The variety in body size and feeding guilds possibly indicates a functioning catenal ecosystem. This knowledge can be beneficial in monitoring and conservation of species in the park. Keywords: catena ecosystem; ephemeral mud wallows; habitat use; mammal variety; Skukuza area; Citation: Janecke, B.B. -

1 the Gearach Booking Terms and Conditions Please Read, Sign
The Gearach Booking Terms and Conditions Please read, sign and date this form and return it to the Gearach along with your deposit. 1 A booking will be provisionally reserved in your name once dates have been agreed by telephone, fax, or e‐mail between both parties. 2 To confirm and secure your reserved dates, 50% of the total fee, together with a signed copy of the booking terms and conditions should be submitted within 7 days. 3 Do not book any hotel or travel arrangements before we notify you that we have received, therefore confirmed, your 50% deposit. We observe a 'first come first served' basis and your agreed dates are only guaranteed once you have received confirmation from ourselves. 4 When a booking is made less than 12 weeks prior to the commencement of the intended visit the total fee will be due within 7 days of the reservation being made. 5 The Gearach reserves the right to cancel your week, without reason, at any time without notice. 6 Payments may be made by any of the following: a. Transfer funds should be sent to the following account: Bank: Barclays Bank PLC Bank Address: Alston Branch, Northumberland, NE6 1PE Account Name: The Gearach Account Number: 60428116 Sort Code: 20‐40‐09 Iban Number: GB85 BARC 2040 0960 4281 16 Swift Number: BARCGB22 b. By UK pounds sterling cheque ‐payable to The Gearach c. By banker's draft in UK pounds sterling payable to The Gearach Any bank charges arising from the transfer of funds will be payable by you. -

Cartridge Displays & Giftware 2018 Trade Catalogue
TMB Designs Cartridge Displays & Giftware 2018 Trade Catalogue 2012 Unit 18 Highgrove Farm Industrial Estate, Pinvin, Nr Pershore, Worcestershire. WR10 2LF. United Kingdom Tel : 0044 (0)1905 840022. Fax: 0044 (0) 1905 840022 Web Site : www.tmbdesigns.co.uk , Email : [email protected] Web Site : www.cartridgedisplays.com , Email : [email protected] Shotgun Cartridge Gallery Listed on these pages are a selection of handmade shotgun cartridge displays and clocks. Mounted in an ornate frame, behind glass on green baize All cartridges are deactivated and are fitted with oiled primers where possible No licences or permits required. SP05 SP04 Paper Cases Display (380 X 480) Paper Cases Clock (380 X 480) SP06 Paper Cases Display containing 12g,16g,20g, 28g, & 410 (505 x 505) Please Note :- SP02, SP03, SP04, SP05, SP06, (Also available in plastic cases SPL07) SP08 & SP09 are also available in plastic case cartridges, but contain mini clays instead of primer tins SP07 Paper Cases British Display. (532 x 532 ) Commercial Sporting Rifle, Military & Pistol Roll turn over cartridges and famous English sporting calibres No licences or permits required. TMB Designs have been producing their range of cartridge displays from their workshop near Pershore in the Worcestershire countryside for the past 16 years. SP10 LEFT Paper Cases Display. Limited Edition Classic British Calibres Containing collectors paper case shotgun rounds CS44 (475 x 362) including 8g,10g,12g,16g,20g,28g,410 & 9mm Containing a range of (550 x 710) calibres produced by -

Managing Deer for Climate, Communities and Conservation
Scottish Environment LINK Managing deer for climate, communities and conservation This publication has been produced by Scottish Environment LINK - a range of organisations involved in land management, forestry, wildlife conservation, cultural heritage, community partnerships, nature education and outdoor recreation. Together, we have many hundreds of thousands of members and supporters. Badenoch & Butterfly Cairngorms John Muir National Trust Nourish Strathspey Conservation Campaign Froglife Trust for Scotland Scotland Conservation Scotland Group Ramblers RSPB Scottish Scottish Scottish Wild Scottish Trees Woodland Scotland Scotland Badgers Raptor Land Group Wildlife for Life Trust Study Group Trust Scotland The position statement is also supported by these non LINK members: Forest Policy North Reforesting Group Harris Trust Scotland © Copyright Scottish Environment LINK 2020 LINK is a Scottish Charity (SC000296) and a Scottish Company Limited by guarantee (SC250899). LINK is core funded by Membership Subscriptions and by grants from Scottish Natural Heritage, Scottish Government and Charitable Trusts. Contact: Mike Daniels , LINK Deer Subgroup Deputy Convener, Head of Land Management, John Muir Trust Cover and inside cover photographs: Red deer (Cervus elaphus) by Peter Cairns 2 Managing deer for climate change and communities Deer management in the 2020s WE RECOGNISE THAT DEER have a vital part to help us meet our climate change targets, expand play in a balanced ecosystem, and we believe that and diversify our woodlands, bring back wildlife, by managing their numbers strategically, we could enhance our landscapes, regenerate our most bring about a wide raft of public benefits such as: fragile rural areas, repopulate our glens and allow communities real influence over how their local n increasing natural woodland cover to strengthen landscapes are managed. -

Mixed-Species Exhibits with Pigs (Suidae)
Mixed-species exhibits with Pigs (Suidae) Written by KRISZTIÁN SVÁBIK Team Leader, Toni’s Zoo, Rothenburg, Luzern, Switzerland Email: [email protected] 9th May 2021 Cover photo © Krisztián Svábik Mixed-species exhibits with Pigs (Suidae) 1 CONTENTS INTRODUCTION ........................................................................................................... 3 Use of space and enclosure furnishings ................................................................... 3 Feeding ..................................................................................................................... 3 Breeding ................................................................................................................... 4 Choice of species and individuals ............................................................................ 4 List of mixed-species exhibits involving Suids ........................................................ 5 LIST OF SPECIES COMBINATIONS – SUIDAE .......................................................... 6 Sulawesi Babirusa, Babyrousa celebensis ...............................................................7 Common Warthog, Phacochoerus africanus ......................................................... 8 Giant Forest Hog, Hylochoerus meinertzhageni ..................................................10 Bushpig, Potamochoerus larvatus ........................................................................ 11 Red River Hog, Potamochoerus porcus ............................................................... -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34. -
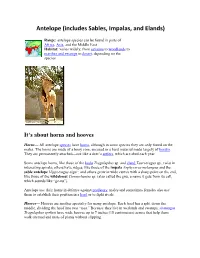
Antelope (Includes Sables, Impalas, and Elands)
Antelope (includes Sables, Impalas, and Elands) Range: antelope species can be found in parts of Africa, Asia, and the Middle East Habitat: varies widely, from savanna to woodlands to marshes and swamps to desert, depending on the species It’s about horns and hooves Horns— All antelope species have horns, although in some species they are only found on the males. The horns are made of a bony core, encased in a hard material made largely of keratin. They are permanently attached—not like a deer’s antlers, which are shed each year. Some antelope horns, like those of the kudu Tragelaphus sp. and eland Taurotragus sp., twist in interesting spirals; others have ridges, like those of the impala Aephyceros melampus and the sable antelope Hippotragus niger; and others grow in wide curves with a sharp point on the end, like those of the wildebeest Connochaetes sp. (also called the gnu, a name it gets from its call, which sounds like “ge-nu”). Antelope use their horns in defense against predators; males and sometimes females also use them to establish their position in a herd or to fight rivals Hooves— Hooves are another specialty for many antelope. Each hoof has a split down the middle, dividing the hoof into two “toes.” Because they live in wetlands and swamps, sitatungas Tragelaphus spekeii have wide hooves up to 7 inches (18 centimeters) across that help them walk on mud and mats of plants without slipping. Nile lechwes Kobus magaceros, which also live in swampy areas, have long, pointed hooves to give them sure footing in the water.