Recent Developments in Cannabis Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cannabinoids in the Pathophysiology of Skin Inflammation
molecules Review Cannabinoids in the Pathophysiology of Skin Inflammation Cristian Scheau 1 , Ioana Anca Badarau 1, Livia-Gratiela Mihai 1, Andreea-Elena Scheau 2, Daniel Octavian Costache 3, Carolina Constantin 4,5, Daniela Calina 6 , Constantin Caruntu 1,7,*, Raluca Simona Costache 8,* and Ana Caruntu 9,10 1 Department of Physiology, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] (C.S.); [email protected] (I.A.B.); [email protected] (L.-G.M.) 2 Department of Radiology and Medical Imaging, Fundeni Clinical Institute, 022328 Bucharest, Romania; [email protected] 3 Department of Dermatology, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 4 Immunology Department, ”Victor Babes” National Institute of Pathology, 050096 Bucharest, Romania; [email protected] 5 Department of Pathology, Colentina University Hospital, 020125 Bucharest, Romania 6 Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, 200349 Craiova, Romania; [email protected] 7 Department of Dermatology, “Prof. N. Paulescu” National Institute of Diabetes, Nutrition and Metabolic Diseases, 011233 Bucharest, Romania 8 Gastroenterology and Internal Medicine Clinic, Carol Davila University Central Emergency Military Hospital, Carol Davila University of Medicine and Pharmacy, 050474 Bucharest, Romania 9 Department of Oral and Maxillofacial Surgery, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 10 Faculty of Medicine, “Titu Maiorescu” University, 031593 Bucharest, Romania * Correspondence: [email protected] (C.C.); [email protected] (R.S.C.); Tel.: +40-745-086-978 (C.C.) Academic Editor: Eric J. Downer Received: 30 December 2019; Accepted: 2 February 2020; Published: 4 February 2020 Abstract: Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component. -
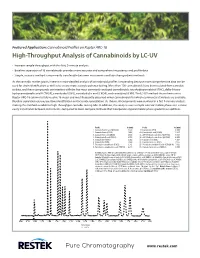
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

Designing Microorganisms for Heterologous Biosynthesis of Cannabinoids Angelaˆ Carvalho1, Esben Halkjær Hansen1, Oliver Kayser2, Simon Carlsen1,∗ and Felix Stehle2
FEMS Yeast Research, 17, 2017, fox037 doi: 10.1093/femsyr/fox037 Advance Access Publication Date: 4 June 2017 Minireview MINIREVIEW Designing microorganisms for heterologous biosynthesis of cannabinoids Angelaˆ Carvalho1, Esben Halkjær Hansen1, Oliver Kayser2, Simon Carlsen1,∗ and Felix Stehle2 1Evolva Biotech A/S, Lersø Parkalle´ 42-44, 2100, Copenhagen, Denmark and 2Laboratory of Technical Biochemistry, Department of Biochemical and Chemical Engineering, TU Dortmund University, Emil-Figge-Str. 66, 44227 Dortmund, Germany ∗Corresponding author: Evolva Biotech A/S, Lersø Parkalle´ 42-44, 2100, Copenhagen, Denmark. Tel: +45-35-200-243; E-mail: [email protected] One sentence summary: In this review, the authors explore the use of synthetic biology as an alternative approach for the synthesis of pharmaceutical cannabinoids in a heterologous host organism. Editor: John Morrissey ABSTRACT During the last decade, the use of medical Cannabis has expanded globally and legislation is getting more liberal in many countries, facilitating the research on cannabinoids. The unique interaction of cannabinoids with the human endocannabinoid system makes these compounds an interesting target to be studied as therapeutic agents for the treatment of several medical conditions. However, currently there are important limitations in the study, production and use of cannabinoids as pharmaceutical drugs. Besides the main constituent tetrahydrocannabinolic acid, the structurally related compound cannabidiol is of high interest as drug candidate. From the more than 100 known cannabinoids reported, most can only be extracted in very low amounts and their pharmacological profile has not been determined. Today, cannabinoids are isolated from the strictly regulated Cannabis plant, and the supply of compounds with sufficient quality is a major problem. -
Marinol Cannabidiol C21H30O2 Trade Name
Cannabinoids are a group of terpenophenolic compounds secreted by Cannabis flowers that provide relief from a wide array of symptoms including, pain, nausea, and inflammation. They operate by imitating the body’s natural endocannabinoids, which activate to maintain internal stability and overall health. When consumed, cannabinoids bind to receptor sites throughout the brain (CB1 receptors) and body (CB2 receptors). Different cannabinoids have different effects based on their binding affinity for each receptor. By targeting specific cannabinoids at these receptors, different types of relief can be achieved. Presently, there are at least 113 different cannabinoids isolated from Cannabis—each exhibiting varied effects. THC Tetrahydrocannabinol C21H30O2 Trade name: Marinol Legal Status: US – Schedule I, Schedule II (as Cesamet), Schedule III (as Marinol) OH CA – Schedule II UK – Class B AU – S8 (controlled) H Psychoactive Tetrahydrocannabinol (THC) is typically the most abundant cannabinoid present in cannabis products on the market today. THC has very high psychoactive characteristics and is associated with the ‘high’ and euphoria experienced when using cannabis products. When smoked or ingested, THC binds to cannabinoid receptors throughout the body and affects memory, O coordination, concentration, pleasure, and time perception. H Medicinal Benefits Analgesic • Anti-nauseant • Appetite Stimulant Reduces Glaucoma Symptoms Sleep Aid • Reduces Anxiety and PTSD Symptoms CBD Cannabidiol C21H30O2 Trade name: Epidiolex OH Legal Status: US – Schedule I CA – Schedule II UK – POM (Perscription only) H AU – S4 (Perscription only) non-psychoactive Cannabidiol (CBD) is a major phytocannabinoid and accounts for up to 40% of the plant’s extract. Due to its lack of psychoactivity and HO non-interference with motor and psychological functions, it is a leading candidate for a wide variety of medical applications. -

What Is Delta-8 THC?? Cannabinoid Chemistry 101
What is Delta-8 THC?? Cannabinoid Chemistry 101 National Conference on Weights and Measures Annual Meeting - Rochester, NY Matthew D. Curran, Ph.D. July 21, 2021 Disclaimer Just to be clear… • I am a chemist and not a lawyer so: • This presentation will not discuss the legal aspects of Δ8-THC or DEA’s current position. • This presentation will not discuss whether Δ8-THC is considered “synthetic” or “naturally occurring.” • This is not a position statement on any issues before the NCWM. • Lastly, this should only be considered a scientific sharing exercise. Florida Department of Agriculture and Consumer Services 2 Cannabis in Florida Cannabis Syllabus • What is Cannabis? • “Mother” Cannabinoid • Decarboxylation • Relationship between CBD and THC • What does “Total” mean? • Dry Weight vs. Wet Weight • What does “Delta-9” mean? • Relationship between “Delta-8” and “Delta-9” • CBD to Delta-8 THC • Cannabinoid Chemistry 202… Florida Department of Agriculture and Consumer Services 3 Cannabis Cannabis • Cannabis sativa is the taxonomic name for the plant. • The concentration of Total Δ9-Tetrahydrocannabinol (Total Δ9-THC) is critical when considering the varieties of Cannabis sativa. • Hemp – (Total Δ9-THC) 0.3% or less • Not really a controversial term, “hemp” • Marijuana/cannabis – (Total Δ9-THC) Greater than 0.3% • Controversial term, “marijuana” • Some states prohibit the use of this term whereas some states have it in their laws. • Some states use the term “cannabis.” • Not italicized • Lower case “c” Florida Department of Agriculture and -

INTRODUCTION One of the Tasks of a Pharmacologist Is the Classification of Drugs
NEUROPSYCHOPHARMACOLOGIC STUDIES OF MARIJUANA: SOME SYNTHETIC AND NATURAL THC DERIVATIVES IN ANIMALS AND MAN* Edward F. Domino Department of Pharmacology, University of Michigan, Ann Arbor 48104 and Lafayette Clinic, Detroit, Mich. 48207 INTRODUCTION One of the tasks of a pharmacologist is the classification of drugs. Marijuana and its derivatives pose some problems. Legally marijuana has been classified as a narcotic, yet pharmacologically it is quite different. Mankind has known about the Cannabis plant from which marijuana and other products are obtained for more than 4,708 years. A considerable body of data is available to describe its gross effects. Over the centuries it has been called a depressant, euphoriant, ine- briant, intoxicant, hallucinogen, psychotomimetic, sedative-hypnotic-anesthetic, and stimulant. Perhaps more than any other drug it is a social irritant that elicits rather remarkable behavior reactions in both users and nonusers. Moreau’ in 1845, employed it to produce a model psychosis, thus initiating the fashion to study hallucinogens as a means of gaining insights into mental illness. Lewin2 classified Cannabis as one of the “phantastica: hallucinating substances.” One text on hallucinogens3 does not even refer to Cannabis, but another does? There are some aspects of the subjective effects of both LSD-25 and Cannabis that are similar, yet the latter produces sedative effects, no significant sympatho- mimetic actions, and no cross-tolerance to LSD-25. Hollister5 reviewed the re- cent findings on the effects in man of marijuana and its putative active ingredient Ag-THC, stressing that current investigators have, for the most part, confirmed the Cannabis-induced clinical syndromes long known to occur. -

The Use of Cannabinoids in Animals and Therapeutic Implications for Veterinary Medicine: a Review
Veterinarni Medicina, 61, 2016 (3): 111–122 Review Article doi: 10.17221/8762-VETMED The use of cannabinoids in animals and therapeutic implications for veterinary medicine: a review L. Landa1, A. Sulcova2, P. Gbelec3 1Faculty of Medicine, Masaryk University, Brno, Czech Republic 2Central European Institute of Technology, Masaryk University, Brno, Czech Republic 3Veterinary Hospital and Ambulance AA Vet, Prague, Czech Republic ABSTRACT: Cannabinoids/medical marijuana and their possible therapeutic use have received increased atten- tion in human medicine during the last years. This increased attention is also an issue for veterinarians because particularly companion animal owners now show an increased interest in the use of these compounds in veteri- nary medicine. This review sets out to comprehensively summarise well known facts concerning properties of cannabinoids, their mechanisms of action, role of cannabinoid receptors and their classification. It outlines the main pharmacological effects of cannabinoids in laboratory rodents and it also discusses examples of possible beneficial use in other animal species (ferrets, cats, dogs, monkeys) that have been reported in the scientific lit- erature. Finally, the article deals with the prospective use of cannabinoids in veterinary medicine. We have not intended to review the topic of cannabinoids in an exhaustive manner; rather, our aim was to provide both the scientific community and clinical veterinarians with a brief, concise and understandable overview of the use of cannabinoids in veterinary -

Preparative Isolation of Cannabinoids from Cannabis Sativa by Centrifugal Partition Chromatography
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/244594577 Preparative Isolation of Cannabinoids from Cannabis sativa by Centrifugal Partition Chromatography Article in Journal of Liquid Chromatography & Related Technologies · December 2004 DOI: 10.1081/JLC-200028170 CITATIONS READS 47 2,645 5 authors, including: Arno Hazekamp Robert Verpoorte Bedrocan BV Leiden University 50 PUBLICATIONS 975 CITATIONS 732 PUBLICATIONS 21,203 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: The effects of inhaled delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on pain relief and subjective effects in fibromyalgia patients View project All content following this page was uploaded by Robert Verpoorte on 20 June 2014. The user has requested enhancement of the downloaded file. Cannabis; extracting the medicine i Arno Hazekamp Cannabis; extracting the medicine Proefschrift Universiteit Leiden ISBN 978-90-9021997-4 Printed by PrintPartners Ipskamp B.V., Amsterdam, The Netherlands Paper cover: Cannabis Pur 100% (250 grams) from Grafisch Papier, The Nederlands. Photo cover: Dutch medicinal cannabis, variety “Bedrocan”. ii Cannabis; extracting the medicine Proefschrift Ter verkrijging van de graad van Doctor aan de Universiteit Leiden, op gezag van de Rector Magnificus prof. mr. P. F. van der Heijden, hoogleraar in de faculteit der Rechtsgeleerdheid, volgens besluit van het College voor Promoties te verdedigen op woensdag 5 september 2007 klokke 15.00 uur door Arno Hazekamp Geboren op 15 maart 1976 te Bilthoven iii Promotiecommissie Promotor Prof. dr. R. Verpoorte Referent Dr. C. Giroud (Institut Universitaire de Médecine Légale, Lausanne, Switzerland) Overige leden Prof. -

Using Dictyostelium Discoideum to Investigate the Mechanism of Action of Cannabigerol
Therapeutic cannabinoids: Using Dictyostelium discoideum to investigate the mechanism of action of cannabigerol Joseph Oddy Research thesis submitted for the degree of Doctor of Philosophy at the Royal Holloway University of London in May 2020 1 Declaration of Authorship I, Joseph Laurence Damstra-Oddy, hereby declare that the work presented in this thesis is my own unless otherwise stated, and that all published work has been acknowledged. Furthermore, I affirm that I have neither fabricated nor falsified the results reported herein. Signed: Date: 22/05/2020 2 Abstract Cannabis has been used to treat many diseases for centuries. Recent medical interest has focused on the potential of cannabinoids to treat diseases such as multiple sclerosis, epilepsy, and cancer where research has focused on investigating cannabidiol (CBD). However, other cannabinoids, such as cannabigerol (CBG), remain poorly characterised and are being explored as potential treatments. The molecular mechanisms by which cannabinoids treat diseases remain unclear, despite suggested targets including adenosine, mTOR, transient receptor potential transporters and cannabinoid receptors. This study aimed to identify molecular mechanisms of CBG, using Dictyostelium discoideum as a model system and translating results to a clinical setting. Initially, a targeted approach was undertaken where the effects of CBG on adenosine transport (and DNA methylation) was investigated. From this approach, CBG elevated DNA methylation in D. discoideum dependent upon adenosine transport via the equilibrative nucleoside transporter 1 (ENT1). In addition, an unbiased approach was taken in which screening of a mutant library for CBG resistance identified inositol polyphosphate multikinase (IPMK), a known regulator of mTOR activity, as a potential target. -

WO 2017/139496 Al 17 August 2017 (17.08.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/139496 Al 17 August 2017 (17.08.2017) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 1/15 (2006.01) C12N 15/52 (2006.01) kind of national protection available): AE, AG, AL, AM, C12N 15/29 (2006.01) C12P 7/40 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C12N 15/31 (2006.01) C12P 7/22 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, PCT/US20 17/0 17246 KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, (22) International Filing Date: MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, ' February 2017 (09.02.2017) NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, (25) Filing Language: English TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, (26) Publication Language: English ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 62/293,050 February 2016 (09.02.2016) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: CEVOLVA BIOTECH, INC. -

The Effects of Cannabinoids on Stress-Coping
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-2021 The Effects of Cannabinoids on Stress-Coping Behaviors and Neuroendocrinological Measures in Chronic Unpredtictable Stress: A Preclinical Systematic Review and Meta-Analysis Noa Reuveni Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Psychology Commons Recommended Citation Reuveni, Noa, "The Effects of Cannabinoids on Stress-Coping Behaviors and Neuroendocrinological Measures in Chronic Unpredtictable Stress: A Preclinical Systematic Review and Meta-Analysis" (2021). All Graduate Theses and Dissertations. 8098. https://digitalcommons.usu.edu/etd/8098 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. THE EFFECTS OF CANNABINOIDS ON STRESS-COPING BEHAVIORS AND NEUROENDOCRINOLOGICAL MEASURES IN CHRONIC UNPREDTICTABLE STRESS: A PRECLINICAL SYSTEMATIC REVIEW AND META-ANALYSIS by Noa Reuveni A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Psychology Approved: __________________ __________________ Sara Freeman, Ph.D. Scott Bates, Ph.D. Co-Major Professor Co-Major Professor __________________ __________________ Diana Meter, Ph.D. Sarah Schwartz, Ph.D. Committee Member Committee Member __________________ __________________ Tyler Renshaw, Ph.D. D. Richard Cutler, Ph.D. Committee Member Interim Vice Provost for Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2021 ii Copyright © Noa Reuveni 2021 All Rights Reserved iii ABSTRACT The Effects of Cannabinoids on Stress-Coping Behaviors and Neuroendocrinological Measures in Chronic Unpredictable Stress: A Preclinical Systematic Review and Meta-Analysis by Noa Reuveni, Master of Science Utah State University, 2021 Major Professors: Dr. -

The Phytocannabinoides from Cannabis Sativa L. an Overview
Hop and Medicinal Plants, Year XXVIII, No. 1-2, 2020 ISSN 2360–0179 print, ISSN 2360–0187 electronic THE PHYTOCANNABINOIDES FROM CANNABIS SATIVA L. AN OVERVIEW ONA Andreea Daniela, Sorin MUNTEAN, Leon MUNTEAN* University of Agricultural Sciences and Veterinary Medicine, Faculty of Agriculture, Crop Science Department 3-5 Mănăstur Street, 400372, Cluj-Napoca, Romania *Corresponding author e-mail: [email protected] Abstract: Cannabis sativa is among the first cultivated plants for producing fibres from the stalks and consumption of the seeds. Later, the medicinal properties were discovered. The oldest written record of using cannabis for the medicinal and recreational purposes is from China. Over the time many experimental researches were carried out that emphasize the importance of cannabinoids for human health. The present paper focused to present an overview of the main cannabinoids synthetized in cannabis plant and their medicinal and therapeutic effects. Cannabis has a complex chemical composition including besides cannabinoids (over 100), also terpenoids, sugars, alkaloids, quinones and stilbenoids. Of major importance are two compounds tetrahydrocannabinol (THC) and cannabidiol (CBD). Over the time different researches pointed out the beneficial effect of cannabinoids on a wide range of diseases such as epilepsy, neurological diseases, mental conditions like anxiety and depression, chronic pains and certain forms of cancer. In addition to the important advantages it must be also paid attention to the psychoactive action of the certain cannabinoids that may produce cognitive disturbances, attention disorders, sever panic, can affect the short-term memory, and slow the reaction time. This deficits induced usually by THC can be moderate by using CBD which reduce the psychoactive effect.