The Effects of Cannabinoids on Stress-Coping
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cannabinoids in the Pathophysiology of Skin Inflammation
molecules Review Cannabinoids in the Pathophysiology of Skin Inflammation Cristian Scheau 1 , Ioana Anca Badarau 1, Livia-Gratiela Mihai 1, Andreea-Elena Scheau 2, Daniel Octavian Costache 3, Carolina Constantin 4,5, Daniela Calina 6 , Constantin Caruntu 1,7,*, Raluca Simona Costache 8,* and Ana Caruntu 9,10 1 Department of Physiology, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] (C.S.); [email protected] (I.A.B.); [email protected] (L.-G.M.) 2 Department of Radiology and Medical Imaging, Fundeni Clinical Institute, 022328 Bucharest, Romania; [email protected] 3 Department of Dermatology, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 4 Immunology Department, ”Victor Babes” National Institute of Pathology, 050096 Bucharest, Romania; [email protected] 5 Department of Pathology, Colentina University Hospital, 020125 Bucharest, Romania 6 Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, 200349 Craiova, Romania; [email protected] 7 Department of Dermatology, “Prof. N. Paulescu” National Institute of Diabetes, Nutrition and Metabolic Diseases, 011233 Bucharest, Romania 8 Gastroenterology and Internal Medicine Clinic, Carol Davila University Central Emergency Military Hospital, Carol Davila University of Medicine and Pharmacy, 050474 Bucharest, Romania 9 Department of Oral and Maxillofacial Surgery, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 10 Faculty of Medicine, “Titu Maiorescu” University, 031593 Bucharest, Romania * Correspondence: [email protected] (C.C.); [email protected] (R.S.C.); Tel.: +40-745-086-978 (C.C.) Academic Editor: Eric J. Downer Received: 30 December 2019; Accepted: 2 February 2020; Published: 4 February 2020 Abstract: Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component. -
Marinol Cannabidiol C21H30O2 Trade Name
Cannabinoids are a group of terpenophenolic compounds secreted by Cannabis flowers that provide relief from a wide array of symptoms including, pain, nausea, and inflammation. They operate by imitating the body’s natural endocannabinoids, which activate to maintain internal stability and overall health. When consumed, cannabinoids bind to receptor sites throughout the brain (CB1 receptors) and body (CB2 receptors). Different cannabinoids have different effects based on their binding affinity for each receptor. By targeting specific cannabinoids at these receptors, different types of relief can be achieved. Presently, there are at least 113 different cannabinoids isolated from Cannabis—each exhibiting varied effects. THC Tetrahydrocannabinol C21H30O2 Trade name: Marinol Legal Status: US – Schedule I, Schedule II (as Cesamet), Schedule III (as Marinol) OH CA – Schedule II UK – Class B AU – S8 (controlled) H Psychoactive Tetrahydrocannabinol (THC) is typically the most abundant cannabinoid present in cannabis products on the market today. THC has very high psychoactive characteristics and is associated with the ‘high’ and euphoria experienced when using cannabis products. When smoked or ingested, THC binds to cannabinoid receptors throughout the body and affects memory, O coordination, concentration, pleasure, and time perception. H Medicinal Benefits Analgesic • Anti-nauseant • Appetite Stimulant Reduces Glaucoma Symptoms Sleep Aid • Reduces Anxiety and PTSD Symptoms CBD Cannabidiol C21H30O2 Trade name: Epidiolex OH Legal Status: US – Schedule I CA – Schedule II UK – POM (Perscription only) H AU – S4 (Perscription only) non-psychoactive Cannabidiol (CBD) is a major phytocannabinoid and accounts for up to 40% of the plant’s extract. Due to its lack of psychoactivity and HO non-interference with motor and psychological functions, it is a leading candidate for a wide variety of medical applications. -

Recent Developments in Cannabis Chemistry
Recent Developments in Cannabis Chemistry BY ALEXANDER T. SHULGIN, Ph.D. The marijuana plant Cannabis sativa contains a bewildering Introduction array of organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono- and sesquiterpenes, carbohy- drates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies. The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitro- gen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of terpene and a substituted resorcinol. Beyond the scope of this present review are such questions as the distribution of these compounds within the plant, the bo- tanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several Reprinted from Journal of Psychedelic Drugs, vol. II, no. 1, 197 1. 397 398 Marijuana: Medical Papers distinct compounds present, and the various preparations and customs of administration. -

INTRODUCTION One of the Tasks of a Pharmacologist Is the Classification of Drugs
NEUROPSYCHOPHARMACOLOGIC STUDIES OF MARIJUANA: SOME SYNTHETIC AND NATURAL THC DERIVATIVES IN ANIMALS AND MAN* Edward F. Domino Department of Pharmacology, University of Michigan, Ann Arbor 48104 and Lafayette Clinic, Detroit, Mich. 48207 INTRODUCTION One of the tasks of a pharmacologist is the classification of drugs. Marijuana and its derivatives pose some problems. Legally marijuana has been classified as a narcotic, yet pharmacologically it is quite different. Mankind has known about the Cannabis plant from which marijuana and other products are obtained for more than 4,708 years. A considerable body of data is available to describe its gross effects. Over the centuries it has been called a depressant, euphoriant, ine- briant, intoxicant, hallucinogen, psychotomimetic, sedative-hypnotic-anesthetic, and stimulant. Perhaps more than any other drug it is a social irritant that elicits rather remarkable behavior reactions in both users and nonusers. Moreau’ in 1845, employed it to produce a model psychosis, thus initiating the fashion to study hallucinogens as a means of gaining insights into mental illness. Lewin2 classified Cannabis as one of the “phantastica: hallucinating substances.” One text on hallucinogens3 does not even refer to Cannabis, but another does? There are some aspects of the subjective effects of both LSD-25 and Cannabis that are similar, yet the latter produces sedative effects, no significant sympatho- mimetic actions, and no cross-tolerance to LSD-25. Hollister5 reviewed the re- cent findings on the effects in man of marijuana and its putative active ingredient Ag-THC, stressing that current investigators have, for the most part, confirmed the Cannabis-induced clinical syndromes long known to occur. -

The Use of Cannabinoids in Animals and Therapeutic Implications for Veterinary Medicine: a Review
Veterinarni Medicina, 61, 2016 (3): 111–122 Review Article doi: 10.17221/8762-VETMED The use of cannabinoids in animals and therapeutic implications for veterinary medicine: a review L. Landa1, A. Sulcova2, P. Gbelec3 1Faculty of Medicine, Masaryk University, Brno, Czech Republic 2Central European Institute of Technology, Masaryk University, Brno, Czech Republic 3Veterinary Hospital and Ambulance AA Vet, Prague, Czech Republic ABSTRACT: Cannabinoids/medical marijuana and their possible therapeutic use have received increased atten- tion in human medicine during the last years. This increased attention is also an issue for veterinarians because particularly companion animal owners now show an increased interest in the use of these compounds in veteri- nary medicine. This review sets out to comprehensively summarise well known facts concerning properties of cannabinoids, their mechanisms of action, role of cannabinoid receptors and their classification. It outlines the main pharmacological effects of cannabinoids in laboratory rodents and it also discusses examples of possible beneficial use in other animal species (ferrets, cats, dogs, monkeys) that have been reported in the scientific lit- erature. Finally, the article deals with the prospective use of cannabinoids in veterinary medicine. We have not intended to review the topic of cannabinoids in an exhaustive manner; rather, our aim was to provide both the scientific community and clinical veterinarians with a brief, concise and understandable overview of the use of cannabinoids in veterinary -
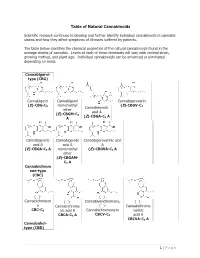
Table of Natural Cannabinoids
Table of Natural Cannabinoids Scientific research continues to develop and further identify individual cannabinoids in cannabis strains and how they affect symptoms of illnesses suffered by patients. The table below identifies the chemical properties of the natural cannabinoids found in the average strains of cannabis. Levels of each of these chemicals will vary with varietal strain, growing method, and plant age. Individual cannabinoids can be enhanced or eliminated depending on need. Cannabigerol- type (CBG) Cannabigerol Cannabigerol Cannabigerovarin (E)-CBG-C monomethyl (E)-CBGV-C 5 Cannabinerolic 3 ether acid A (E)-CBGM-C 5 (Z)-CBGA-C A A 5 Cannabigerolic Cannabigerolic Cannabigerovarinic acid acid A acid A A (E)-CBGA-C5 A monomethyl (E)-CBGVA-C3 A ether (E)-CBGAM- C5 A Cannabichrom ene-type (CBC) (±)- (±)- Cannabichromen (±)- Cannabivarichromene, (±)- e Cannabichrome (±)- Cannabichrome CBC-C5 nic acid A Cannabichromevarin varinic CBCA-C5 A CBCV-C3 acid A CBCVA-C3 A Cannabidiol- type (CBD) 1 | Page (−)-Cannabidiol Cannabidiol Cannabidiol-C4 (−)- Cannabidiorc CBD-C5 momomethyl CBD-C4 Cannabidivarin ol ether CBDV-C3 CBD-C1 CBDM-C5 Cannabidiolic Cannabidivarini acid c acid CBDA-C5 CBDVA-C3 Cannabinodiol- type (CBND) Cannabinodiol Cannabinodivar CBND-C5 in CBND-C3 Tetrahydrocan nabinol-type (THC) 9 9 9 Δ - Δ - Δ - Δ9- Tetrahydrocanna Tetrahydrocan Tetrahydrocannabivarin 9 Tetrahydrocan binol nabinol-C4 Δ -THCV-C3 9 9 nabiorcol Δ -THC-C5 Δ -THC-C4 9 Δ -THCO-C1 9 9 Δ -Tetrahydro- Δ9-Tetrahydro- Δ -Tetrahydro- Δ9-Tetrahydro- cannabinolic -

Cannabinoid Receptor Antagonists Counteract Sensorimotor Gating Deficits in the Phencyclidine Model of Psychosis
Neuropsychopharmacology (2007) 32, 2098–2107 & 2007 Nature Publishing Group All rights reserved 0893-133X/07 $30.00 www.neuropsychopharmacology.org Cannabinoid Receptor Antagonists Counteract Sensorimotor Gating Deficits in the Phencyclidine Model of Psychosis ,1 2,3 4 5 3,6 Martina Ballmaier* , Marco Bortolato , Cristina Rizzetti , Michele Zoli , GianLuigi Gessa , 1,6 4,6 Andreas Heinz and PierFranco Spano 1Department of Psychiatry and Psychotherapy, Charite´ University Medicine, Campus Mitte, Berlin, Germany; 2Department of Pharmacology, 3 4 University of California, Irvine, USA; Department of Neuroscience, Bernard B Brodie, University of Cagliari, Cagliari, Italy; Department of 5 Biomedical Sciences and Biotechnologies, Brescia University Medical School, Brescia, Italy; Department of Biomedical Sciences, University of Modena and Reggio Emilia, Modena, Italy Clinical and laboratory findings suggest that cannabinoids and their receptors are implicated in schizophrenia. The role of cannabinoids in schizophrenia remains however poorly understood, as data are often contradictory. The primary aim of this study was to investigate whether the cannabinoid CB1 receptor antagonists rimonabant and AM251 are able to reverse deficits of sensorimotor gating induced by phencyclidine and to mimic the ‘atypical’ antipsychotic profile of clozapine. The prepulse inhibition (PPI) of the startle reflex was used to measure deficits of sensorimotor gating. PPI-disruptive effects of phencyclidine and their antagonism by rimonabant, AM251, and clozapine were studied in rats. The effects of rimonabant were carefully examined taking into account dose ranges, vehicle, and route of administration. We also examined the ability of rimonabant to reduce the PPI-disruptive effects of dizocilpine and apomorphine. Rimonabant as well as AM251 significantly counteracted the phencyclidine-disruptive model of PPI, comparable to the restoring effect of clozapine; no augmentation effect was observed with rimonabant and clozapine as cotreatment. -

Structure-Activity Relationships of the Cannabinoids
RESEARCH ANALYSIS and UTILIZATION SYSTEM Structure-Activity Relationships of the Cannabinoids DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration Structure-Activity Relationships of the Cannabinoids Editors: Rao S. Rapaka, Ph.D. Division of Preclinical Research National Institute on Drug Abuse Alexandros Makriyannis, Ph.D. School of Pharmacy and Institute of Materials Science University of Connecticut and F. Bitter National Magnet Laboratory Massachusetts Institute of Technology NIDA Research Monograph 79 A RAUS Review Report 1987 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse 5600 Fishers Lane Rockville, Maryland 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 NIDA Research Monographs are prepared by the research divisions of the National Institute on Drug Abuse and published by its Office of Science. The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. MARY L. JACOBSON Temple University School of Medicine National Federation of Parents for Philadelphia, Pennsylvania Drug-Free Youth Omaha, Nebraska SYDNEY ARCHER, Ph.D. Rensselaer Polytechnic lnstitute Troy, New York REESE T. JONES, M.D. Langley Porter Neuropsychiatric Institute RICHARD E. BELLEVILLE, Ph.D. San Francisco, California NB Associates. Health Sciences Rockville, Maryland DENISE KANDEL, Ph.D. KARST J. BESTEMAN College of Physicians and Surgeons of Alcohol and Drug Problems Association Columbia University of North America New York, New York Washington, D.C. -

INFORMATION to USERS the Most Advanced Technology Has Been Used to Photo Graph and Reproduce This Manuscript from the Microfilm Master
Synthesis of cannabidiol stereoisomers and analogs as potential anticonvulsant agents. Item Type text; Dissertation-Reproduction (electronic) Authors Shah, Vibhakar Jayantilal. Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 02/10/2021 14:16:58 Link to Item http://hdl.handle.net/10150/184523 INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely eve~~t that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginni'ng at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. -

Interaction of Cannabis and General Anaesthetic Agents in Mice G.B
Br. J. Pharmac. (1974), 50, 593-599 INTERACTION OF CANNABIS AND GENERAL ANAESTHETIC AGENTS IN MICE G.B. CHESHER, D.M. JACKSON & G.A. STARMER Department of Pharmacology, University of Sydney, Sydney 2006, Australia 1 A cannabis extract (I) (in a concentration equivalent to 10 mg A9-tetrahydro- cannabinol(THC)/kg) prolonged pentobarbitone anaesthesia in mice maximally 20 min to 2 h after medication. The effect was still significant after 8 h, but less than at 2 hours. 2 The cannabis extract (1) (equivalent to 10 mg A9-THC/kg) prolonged both pentobarbitone and ether anaesthesia in mice when administered 20 min before the anaesthetic. After eight consecutive daily doses of cannabis, the pentobarbitone anaesthesia was still significantly longer than a control group, while ether anaesthesia was not significantly prolonged. 3 A second cannabis extract (II) with a different ratio of cannabinoids (also administered in dosage equivalent to 10 mg A9-THC/kg) failed to affect pentobarbitone anaesthesia in mice. This extract presented about 4% the dose of cannabidiol as extract I. 4 A8-THC, A9-THC and cannabidiol prolonged pentobarbitone anaesthesia with cannabidiol being generally more active than A 9 -THC. Cannabinol ( 10 mg/kg) was inactive. 5 The effects of cannabidiol and A9-THC were found to be additive, and there was a consistent trend for cannabinol to reduce the effectiveness of A9-THC and cannabidiol when given in combination. 6 Premedication with phenoxybenzamine, phentolamine, propranolol, iproniazid, protripty- line, desipramine, reserpine, ai-methyl tyrosine or parachlorophenylalanine did not affect the extract I-induced prolongation of pentobarbitone anaesthesia. 7 It is concluded that cannabis may affect pentobarbitone and ether anaesthesia in mice at least partially by a direct depressant effect, and that the cannabis-induced prolongation of anaesthesia is probably unrelated to any effect on central 5-hydroxytryptamine or catechol- amine neurones. -

Advisory Council on the Misuse of Drugs
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Working Group Secretary: Linsey Urquhart 1st Floor (NE) Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 8846 Email: [email protected] Sarah Newton MP Minister for Vulnerability, Safeguarding and Countering Extremism Home Office 2 Marsham Street London SW1P 4DF 16 December 2016 Dear Minister, Re: ACMD Report - Phytocannabinoids On 27 April 2016 I wrote to the then Home Secretary outlining the annual work programme for the Advisory Council on the Misuse of Drugs (ACMD). This included a new working group to review the generic definition for plant cannabinoids (phytocannabinoids) in the Misuse of Drugs Act 1971 (MDA). This work followed the 2013 commission from the Home Office to the ACMD to keep under review the generic definitions in the Act and an appreciation that the literature available on the psychoactivity of phytocannabinoids has increased since the definition was first conceived. We are pleased to inform you that this work has now been completed and provide the attached report as a presentation of our findings. Key Findings • A review of the literature has found that 97 phytocannabinoids have been identified so far. • Under the agreed interpretation of the generic definition, 12 of these phytocannabinoids would be considered controlled under the MDA. 1 • There is sufficient evidence available for three of these controlled phytocannabinoids to conclude that they are psychoactive. • There is one controlled phytocannabinoid (delta-9-tetrahydrocannabinol-C3, THCV) for which there is conflicting evidence of limited psychoactivity. • For the remaining eight controlled phytocannabinoids, there was insufficient evidence available to determine psychoactivity or the absence thereof. -

Cannabinoids Using ALZET Osmotic Pumps
ALZET® Bibliography References on the Administration of Cannabinoids Using ALZET Osmotic Pumps Q5966: P. Weydt. Mechanisms and Modifiers of Energy Metabolism in ALS and Huntington Disease. Open Access Repositorium der Universität Ulm 2016; ALZET Comments: Cannabinol; PEG 400; SC; Mice; 2004; 4 weeks; animal info (SOD 1 transgenic); pumps replaced every 28 days; Therapeutic indication (amyotrophic lateral sclerosis); Dose (5 mg/kg);. Q3610: E. J. Rahn, et al. Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Molecular Pain 2014;10(U1-U19 ALZET Comments: AM1710; Taxol-WIN-55212-2; AM251; AM630; DMSO; PEG 400; SC; Rat; 2ML4; 28 days; Controls received mp w/ saline; animal info (male, Sprague Dawley, 300-400g); functionality of mp verified by residual volume; 50% PEG 400 used; 50% DMSO used; Multiple pumps per animal (2); stress/adverse reaction: (see pg.15); behavioral testing (mechanical threshold, cold withdrawal, locomotor activity); AM1710 is a cannabilactone CB2-selective agonist; pumps removed on day 22; WIN55,212-2 is a CB1/CB2 agonist;. Q4814: Heloísa Helena Vilela Costa, et al. Continuous central infusion of cannabinoid receptor agonist WIN 55,212-2 decreases maternal care in lactating rats: Consequences for fear conditioning in adulthood males. Behav. Brain Res 2013;257(31-38 ALZET Comments: WIN-55212-2; NaCl; tween, DMSO; CSF/CNS; Rat; 1007D; 7 days; Controls received mp w/ vehicle; animal info (female, Wistar, 9 weeks old); 10% DMSO used; dose-response (pg 34); behavioral testing (fear conditioning, maternal behavior); teratology; “The continuous infusion of WIN in the CNS using an osmotic minipump in lactating dams eliminates the possibility that WIN affects the behavior of the offspring when offered in the milk.