Table of Natural Cannabinoids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Drugs Manual September 2019
Drug Enforcement Administration Office of Forensic Sciences Analysis of Drugs Manual September 2019 Date Posted: 10/23/2019 Analysis of Drugs Manual Revision: 4 Issue Date: September 5, 2019 Effective Date: September 9, 2019 Approved By: Nelson A. Santos Table of Contents CHAPTER 1 – QUALITY ASSURANCE ......................................................................... 3 CHAPTER 2 – EVIDENCE ANALYSIS ......................................................................... 93 CHAPTER 3 – FIELD ASSISTANCE .......................................................................... 165 CHAPTER 4 – FINGERPRINT AND SPECIAL PROGRAMS ..................................... 179 Appendix 1A – Definitions ........................................................................................... 202 Appendix 1B – Acronyms and Abbreviations .............................................................. 211 Appendix 1C – Instrument Maintenance Schedule ..................................................... 218 Appendix 1D – Color Test Reagent Preparation and Procedures ............................... 224 Appendix 1E – Crystal and Precipitate Test Reagent Preparation and Procedures .... 241 Appendix 1F – Thin Layer Chromatography................................................................ 250 Appendix 1G – Qualitative Method Modifications ........................................................ 254 Appendix 1H – Analytical Supplies and Services ........................................................ 256 Appendix 2A – Random Sampling Procedures -

Targeting the Endocannabinoid System to Reduce Nociception
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 Targeting the endocannabinoid system to reduce nociception Lamont Booker Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2419 This Dissertation is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Targeting the Endocannabinoid System to Reduce Nociception A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Virginia Commonwealth University. By Lamont Booker Bachelor’s of Science, Fayetteville State University 2003 Master’s of Toxicology, North Carolina State University 2005 Director: Dr. Aron H. Lichtman, Professor, Pharmacology & Toxicology Virginia Commonwealth University Richmond, Virginia April 2011 Acknowledgements The author wishes to thank several people. I like to thank my advisor Dr. Aron Lichtman for taking a chance and allowing me to work under his guidance. He has been a great influence not only with project and research direction, but as an excellent example of what a mentor should be (always willing to listen, understanding the needs of each student/technician, and willing to provide a hand when available). Additionally, I like to thank all of my committee members (Drs. Galya Abdrakmanova, Francine Cabral, Sandra Welch, Mike Grotewiel) for your patience and willingness to participate as a member. Our term together has truly been memorable! I owe a special thanks to Sheryol Cox, and Dr. -

Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System
International Journal of Molecular Sciences Review Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System Shenglong Zou and Ujendra Kumar * Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC V6T 1Z4, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-604-827-3660; Fax: +1-604-822-3035 Received: 9 February 2018; Accepted: 11 March 2018; Published: 13 March 2018 Abstract: The biological effects of cannabinoids, the major constituents of the ancient medicinal plant Cannabis sativa (marijuana) are mediated by two members of the G-protein coupled receptor family, cannabinoid receptors 1 (CB1R) and 2. The CB1R is the prominent subtype in the central nervous system (CNS) and has drawn great attention as a potential therapeutic avenue in several pathological conditions, including neuropsychological disorders and neurodegenerative diseases. Furthermore, cannabinoids also modulate signal transduction pathways and exert profound effects at peripheral sites. Although cannabinoids have therapeutic potential, their psychoactive effects have largely limited their use in clinical practice. In this review, we briefly summarized our knowledge of cannabinoids and the endocannabinoid system, focusing on the CB1R and the CNS, with emphasis on recent breakthroughs in the field. We aim to define several potential roles of cannabinoid receptors in the modulation of signaling pathways and in association with several pathophysiological conditions. We believe that the therapeutic significance of cannabinoids is masked by the adverse effects and here alternative strategies are discussed to take therapeutic advantage of cannabinoids. Keywords: cannabinoid; endocannabinoid; receptor; signaling; central nervous system 1. Introduction The plant Cannabis sativa, better known as marijuana, has long been used for medical purpose throughout human history. -

Development and Validation of an Ultra-Fast and Sensitive Microflow
Drug Testing Research article and Analysis Received: 6 June 2016 Revised: 9 July 2016 Accepted: 10 July 2016 Published online in Wiley Online Library: 10 August 2016 (www.drugtestinganalysis.com) DOI 10.1002/dta.2042 Development and validation of an ultra-fast and sensitive microflow liquid chromatography- tandem mass spectrometry (MFLC-MS/MS) method for quantification of LSD and its metabolites in plasma and application to a controlled LSD administration study in humans Andrea E. Steuer,a* Michael Poetzsch,a Lorena Stock,a Lisa Eisenbeiss,a Yasmin Schmid,b Matthias E. Liechtib and Thomas Kraemera Lysergic acid diethylamide (LSD) is a semi-synthetic hallucinogen that has gained popularity as a recreational drug and has been investigated as an adjunct to psychotherapy. Analysis of LSD represents a major challenge in forensic toxicology due to its insta- bility, low drug concentrations, and short detection windows in biological samples. A new, fast, and sensitive microflow liquid chromatography (MFLC) tandem mass spectrometry method for the validated quantification of LSD, iso-LSD, 2-oxo 3-hydroxy- LSD (oxo-HO-LSD), and N-desmethyl-LSD (nor-LSD) was developed in plasma and applied to a controlled pharmacokinetic (PK) study in humans to test whether LSD metabolites would offer for longer detection windows. Five hundred microlitres of plasma were extracted by solid phase extraction. Analysis was performed on a Sciex Eksigent MFLC system coupled to a Sciex 5500 QTrap. The method was validated according to (inter)-national guidelines. MFLC allowed for separation of the mentioned analytes within 3 minutes and limits of quantification of 0.01 ng/mL. -

N-Acyl-Dopamines: Novel Synthetic CB1 Cannabinoid-Receptor Ligands
Biochem. J. (2000) 351, 817–824 (Printed in Great Britain) 817 N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo Tiziana BISOGNO*, Dominique MELCK*, Mikhail Yu. BOBROV†, Natalia M. GRETSKAYA†, Vladimir V. BEZUGLOV†, Luciano DE PETROCELLIS‡ and Vincenzo DI MARZO*1 *Istituto per la Chimica di Molecole di Interesse Biologico, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy, †Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, R. A. S., 16/10 Miklukho-Maklaya Str., 117871 Moscow GSP7, Russia, and ‡Istituto di Cibernetica, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy We reported previously that synthetic amides of polyunsaturated selectivity for the anandamide transporter over FAAH. AA-DA fatty acids with bioactive amines can result in substances that (0.1–10 µM) did not displace D1 and D2 dopamine-receptor interact with proteins of the endogenous cannabinoid system high-affinity ligands from rat brain membranes, thus suggesting (ECS). Here we synthesized a series of N-acyl-dopamines that this compound has little affinity for these receptors. AA-DA (NADAs) and studied their effects on the anandamide membrane was more potent and efficacious than anandamide as a CB" transporter, the anandamide amidohydrolase (fatty acid amide agonist, as assessed by measuring the stimulatory effect on intra- hydrolase, FAAH) and the two cannabinoid receptor subtypes, cellular Ca#+ mobilization in undifferentiated N18TG2 neuro- CB" and CB#. NADAs competitively inhibited FAAH from blastoma cells. This effect of AA-DA was counteracted by the l µ N18TG2 cells (IC&! 19–100 M), as well as the binding of the CB" antagonist SR141716A. -

Cannabinoids in the Pathophysiology of Skin Inflammation
molecules Review Cannabinoids in the Pathophysiology of Skin Inflammation Cristian Scheau 1 , Ioana Anca Badarau 1, Livia-Gratiela Mihai 1, Andreea-Elena Scheau 2, Daniel Octavian Costache 3, Carolina Constantin 4,5, Daniela Calina 6 , Constantin Caruntu 1,7,*, Raluca Simona Costache 8,* and Ana Caruntu 9,10 1 Department of Physiology, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] (C.S.); [email protected] (I.A.B.); [email protected] (L.-G.M.) 2 Department of Radiology and Medical Imaging, Fundeni Clinical Institute, 022328 Bucharest, Romania; [email protected] 3 Department of Dermatology, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 4 Immunology Department, ”Victor Babes” National Institute of Pathology, 050096 Bucharest, Romania; [email protected] 5 Department of Pathology, Colentina University Hospital, 020125 Bucharest, Romania 6 Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, 200349 Craiova, Romania; [email protected] 7 Department of Dermatology, “Prof. N. Paulescu” National Institute of Diabetes, Nutrition and Metabolic Diseases, 011233 Bucharest, Romania 8 Gastroenterology and Internal Medicine Clinic, Carol Davila University Central Emergency Military Hospital, Carol Davila University of Medicine and Pharmacy, 050474 Bucharest, Romania 9 Department of Oral and Maxillofacial Surgery, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 10 Faculty of Medicine, “Titu Maiorescu” University, 031593 Bucharest, Romania * Correspondence: [email protected] (C.C.); [email protected] (R.S.C.); Tel.: +40-745-086-978 (C.C.) Academic Editor: Eric J. Downer Received: 30 December 2019; Accepted: 2 February 2020; Published: 4 February 2020 Abstract: Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component. -

Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma
cells Article Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma Tamara T. Lah 1,2,3,*, Metka Novak 1, Milagros A. Pena Almidon 4, Oliviero Marinelli 4 , Barbara Žvar Baškoviˇc 1, Bernarda Majc 1,3, Mateja Mlinar 1, Roman Bošnjak 5, Barbara Breznik 1 , Roby Zomer 6 and Massimo Nabissi 4 1 Department of Genetic Toxicology and Cancer Biology, National Institute of Biology, 1000 Ljubljana, Slovenia; [email protected] (M.N.); [email protected] (B.Ž.B.); [email protected] (B.M.); [email protected] (M.M.); [email protected] (B.B.) 2 Faculty of Chemistry and Chemical Technology, University of Ljubljana, 1000 Ljubljana, Slovenia 3 Jožef Stefan International Postgraduate School, 1000 Ljubljana, Slovenia 4 School of Pharmacy, Experimental Medicine Section, University of Camerino, 62032 Camerino, Italy; [email protected] (M.A.P.A.); [email protected] (O.M.); [email protected] (M.N.) 5 Department of Neurosurgery, University Medical Centre Ljubljana, 1000 Ljubljana, Slovenia; [email protected] 6 MGC Pharmaceuticals d.o.o., 1000 Ljubljana, Slovenia; [email protected] * Correspondence: [email protected]; Tel.: +386-41-651-629 Simple Summary: Among primary brain tumours, glioblastoma is the most aggressive. As early relapses are unavoidable despite standard-of-care treatment, the cannabinoids delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) alone or in combination have been suggested as a combined treatment strategy for glioblastomas. However, the known psychoactive effects of THC hamper its medical applications in these patients with potential cognitive impairment due to the progression of the Citation: Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; disease. -

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -

A Dissertation Entitled Uncovering Cannabinoid Signaling in C. Elegans
A Dissertation Entitled Uncovering Cannabinoid Signaling in C. elegans: A New Platform to Study the Effects of Medicinal Cannabis By Mitchell Duane Oakes Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology ________________________________________ Dr. Richard Komuniecki, Committee Chair _______________________________________ Dr. Bruce Bamber, Committee Member ________________________________________ Dr. Patricia Komuniecki, Committee Member ________________________________________ Dr. Robert Steven, Committee Member ________________________________________ Dr. Ajith Karunarathne, Committee Member ________________________________________ Dr. Jianyang Du, Committee Member ________________________________________ Dr. Amanda Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo August 2018 Copyright 2018, Mitchell Duane Oakes This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Uncovering Cannabinoid Signaling in C. elegans: A New Platform to Study the Effects of Medical Cannabis By Mitchell Duane Oakes Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology The University of Toledo August 2018 Cannabis or marijuana, a popular recreational drug, alters sensory perception and exerts a range of medicinal benefits. The present study demonstrates that C. elegans exposed to -

Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution
pharmaceutics Article Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution Ema Kosovi´c 1,2 , David Sýkora 2 and Martin Kuchaˇr 3,* 1 Institute of Chemical Process Fundamentals of CAS v.v.i., Rozvojová 135, 16502 Prague, Czech Republic; [email protected] 2 Department of Analytical Chemistry, University of Chemistry and Technology Prague, Technická 5, 16628 Prague, Czech Republic; [email protected] 3 Forensic Laboratory of Biologically Active Substances, Department of Chemistry of Natural Compounds, University of Chemistry and Technology Prague, Technická 5, 16628 Prague, Czech Republic * Correspondence: [email protected] Abstract: Stability studies represent an essential component of pharmaceutical development, en- abling critical evaluation of the therapeutic potential of an active pharmaceutical ingredient (API) or a final pharmaceutical product under the influence of various environmental factors. The aim of the present study was to investigate the chemical stability of cannabidiol (CBD) in the form of a solid powder (hereinafter referred to as CBD powder) and also dissolved in sunflower oil. We performed stress studies in accordance with the International Conference on Harmonization (ICH) guidelines, where 5 mg of marketed CBD in the form of a solid powder and in form of oil solution were exposed for 7 and 14, 30, 60, 90, 180, 270, and 365 days to precisely defined temperature and humidity conditions, 25 ◦C ± 2 ◦C/60% RH ± 5% and 40 ◦C ± 2 ◦C/75% RH ± 5% in both open and closed vials in the dark. CBD powder was significantly more stable than CBD in oil solution. Such finding is important because CBD is often administered dissolved in oil matrix in practice due to Citation: Kosovi´c,E.; Sýkora, D.; very good bioavailability. -
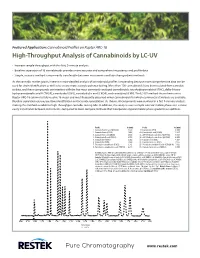
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

CBD (Cannabidiol)
TRANSPORTATION RESEARCH BOARD Driving Toward the Truth - Dispelling the Myths About Cannabis Products February 10, 2021 @NASEMTRB #TRBwebinar PDH Certification The Transportation Research Board has met the standards and Information: requirements of the Registered Continuing Education Providers •1.5 Professional Development Program. Credit earned on completion Hour (PDH) – see follow-up of this program will be reported to email for instructions RCEP. A certificate of completion will •You must attend the entire be issued to participants that have registered and attended the entire webinar to be eligible to receive session. As such, it does not include PDH credits content that may be deemed or •Questions? Contact Reggie construed to be an approval or Gillum at [email protected] endorsement by RCEP. #TRBwebinar Learning Objectives 1. Identify impacts of the Farm Bill on use of THC and CBD products 2. Describe the toxicology of THC and CBD products 3. Discuss how THC and CBD products affect driving performance and crash risk #TRBwebinar TRB Standing Committee on Impairment in Transportation (ACS50) TRB Webinar: Driving Toward the Truth - Dispelling the Myths About Cannabis Products Dr. Barry K. Logan Executive Director, Center for Forensic Science Research and Education (CFSRE); Senior Vice President of Forensic Sciences, and Chief Scientist at NMS Labs Michelle Peace, Ph.D. Associate Professor and PI, Laboratory for Forensic Toxicology Research Department of Forensic Science, Virginia Commonwealth University Dr. Darrin Grondel Vice President,