Critical Appraisal of the Potential Use of Cannabinoids in Cancer Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
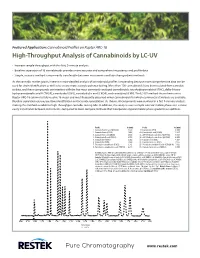
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

Recent Developments in Cannabis Chemistry
Recent Developments in Cannabis Chemistry BY ALEXANDER T. SHULGIN, Ph.D. The marijuana plant Cannabis sativa contains a bewildering Introduction array of organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono- and sesquiterpenes, carbohy- drates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies. The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitro- gen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of terpene and a substituted resorcinol. Beyond the scope of this present review are such questions as the distribution of these compounds within the plant, the bo- tanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several Reprinted from Journal of Psychedelic Drugs, vol. II, no. 1, 197 1. 397 398 Marijuana: Medical Papers distinct compounds present, and the various preparations and customs of administration. -

Preparative Isolation of Cannabinoids from Cannabis Sativa by Centrifugal Partition Chromatography
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/244594577 Preparative Isolation of Cannabinoids from Cannabis sativa by Centrifugal Partition Chromatography Article in Journal of Liquid Chromatography & Related Technologies · December 2004 DOI: 10.1081/JLC-200028170 CITATIONS READS 47 2,645 5 authors, including: Arno Hazekamp Robert Verpoorte Bedrocan BV Leiden University 50 PUBLICATIONS 975 CITATIONS 732 PUBLICATIONS 21,203 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: The effects of inhaled delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on pain relief and subjective effects in fibromyalgia patients View project All content following this page was uploaded by Robert Verpoorte on 20 June 2014. The user has requested enhancement of the downloaded file. Cannabis; extracting the medicine i Arno Hazekamp Cannabis; extracting the medicine Proefschrift Universiteit Leiden ISBN 978-90-9021997-4 Printed by PrintPartners Ipskamp B.V., Amsterdam, The Netherlands Paper cover: Cannabis Pur 100% (250 grams) from Grafisch Papier, The Nederlands. Photo cover: Dutch medicinal cannabis, variety “Bedrocan”. ii Cannabis; extracting the medicine Proefschrift Ter verkrijging van de graad van Doctor aan de Universiteit Leiden, op gezag van de Rector Magnificus prof. mr. P. F. van der Heijden, hoogleraar in de faculteit der Rechtsgeleerdheid, volgens besluit van het College voor Promoties te verdedigen op woensdag 5 september 2007 klokke 15.00 uur door Arno Hazekamp Geboren op 15 maart 1976 te Bilthoven iii Promotiecommissie Promotor Prof. dr. R. Verpoorte Referent Dr. C. Giroud (Institut Universitaire de Médecine Légale, Lausanne, Switzerland) Overige leden Prof. -

The Phytocannabinoides from Cannabis Sativa L. an Overview
Hop and Medicinal Plants, Year XXVIII, No. 1-2, 2020 ISSN 2360–0179 print, ISSN 2360–0187 electronic THE PHYTOCANNABINOIDES FROM CANNABIS SATIVA L. AN OVERVIEW ONA Andreea Daniela, Sorin MUNTEAN, Leon MUNTEAN* University of Agricultural Sciences and Veterinary Medicine, Faculty of Agriculture, Crop Science Department 3-5 Mănăstur Street, 400372, Cluj-Napoca, Romania *Corresponding author e-mail: [email protected] Abstract: Cannabis sativa is among the first cultivated plants for producing fibres from the stalks and consumption of the seeds. Later, the medicinal properties were discovered. The oldest written record of using cannabis for the medicinal and recreational purposes is from China. Over the time many experimental researches were carried out that emphasize the importance of cannabinoids for human health. The present paper focused to present an overview of the main cannabinoids synthetized in cannabis plant and their medicinal and therapeutic effects. Cannabis has a complex chemical composition including besides cannabinoids (over 100), also terpenoids, sugars, alkaloids, quinones and stilbenoids. Of major importance are two compounds tetrahydrocannabinol (THC) and cannabidiol (CBD). Over the time different researches pointed out the beneficial effect of cannabinoids on a wide range of diseases such as epilepsy, neurological diseases, mental conditions like anxiety and depression, chronic pains and certain forms of cancer. In addition to the important advantages it must be also paid attention to the psychoactive action of the certain cannabinoids that may produce cognitive disturbances, attention disorders, sever panic, can affect the short-term memory, and slow the reaction time. This deficits induced usually by THC can be moderate by using CBD which reduce the psychoactive effect. -

Table of Natural Cannabinoids
Table of Natural Cannabinoids Scientific research continues to develop and further identify individual cannabinoids in cannabis strains and how they affect symptoms of illnesses suffered by patients. The table below identifies the chemical properties of the natural cannabinoids found in the average strains of cannabis. Levels of each of these chemicals will vary with varietal strain, growing method, and plant age. Individual cannabinoids can be enhanced or eliminated depending on need. Cannabigerol- type (CBG) Cannabigerol Cannabigerol Cannabigerovarin (E)-CBG-C monomethyl (E)-CBGV-C 5 Cannabinerolic 3 ether acid A (E)-CBGM-C 5 (Z)-CBGA-C A A 5 Cannabigerolic Cannabigerolic Cannabigerovarinic acid acid A acid A A (E)-CBGA-C5 A monomethyl (E)-CBGVA-C3 A ether (E)-CBGAM- C5 A Cannabichrom ene-type (CBC) (±)- (±)- Cannabichromen (±)- Cannabivarichromene, (±)- e Cannabichrome (±)- Cannabichrome CBC-C5 nic acid A Cannabichromevarin varinic CBCA-C5 A CBCV-C3 acid A CBCVA-C3 A Cannabidiol- type (CBD) 1 | Page (−)-Cannabidiol Cannabidiol Cannabidiol-C4 (−)- Cannabidiorc CBD-C5 momomethyl CBD-C4 Cannabidivarin ol ether CBDV-C3 CBD-C1 CBDM-C5 Cannabidiolic Cannabidivarini acid c acid CBDA-C5 CBDVA-C3 Cannabinodiol- type (CBND) Cannabinodiol Cannabinodivar CBND-C5 in CBND-C3 Tetrahydrocan nabinol-type (THC) 9 9 9 Δ - Δ - Δ - Δ9- Tetrahydrocanna Tetrahydrocan Tetrahydrocannabivarin 9 Tetrahydrocan binol nabinol-C4 Δ -THCV-C3 9 9 nabiorcol Δ -THC-C5 Δ -THC-C4 9 Δ -THCO-C1 9 9 Δ -Tetrahydro- Δ9-Tetrahydro- Δ -Tetrahydro- Δ9-Tetrahydro- cannabinolic -

Structure-Activity Relationships of the Cannabinoids
RESEARCH ANALYSIS and UTILIZATION SYSTEM Structure-Activity Relationships of the Cannabinoids DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration Structure-Activity Relationships of the Cannabinoids Editors: Rao S. Rapaka, Ph.D. Division of Preclinical Research National Institute on Drug Abuse Alexandros Makriyannis, Ph.D. School of Pharmacy and Institute of Materials Science University of Connecticut and F. Bitter National Magnet Laboratory Massachusetts Institute of Technology NIDA Research Monograph 79 A RAUS Review Report 1987 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse 5600 Fishers Lane Rockville, Maryland 20857 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 NIDA Research Monographs are prepared by the research divisions of the National Institute on Drug Abuse and published by its Office of Science. The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. Its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. MARY L. JACOBSON Temple University School of Medicine National Federation of Parents for Philadelphia, Pennsylvania Drug-Free Youth Omaha, Nebraska SYDNEY ARCHER, Ph.D. Rensselaer Polytechnic lnstitute Troy, New York REESE T. JONES, M.D. Langley Porter Neuropsychiatric Institute RICHARD E. BELLEVILLE, Ph.D. San Francisco, California NB Associates. Health Sciences Rockville, Maryland DENISE KANDEL, Ph.D. KARST J. BESTEMAN College of Physicians and Surgeons of Alcohol and Drug Problems Association Columbia University of North America New York, New York Washington, D.C. -

(12) Patent Application Publication (10) Pub. No.: US 2013/0059018 A1 Parollaro Et Al
US 2013 005901 8A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0059018 A1 ParollarO et al. (43) Pub. Date: Mar. 7, 2013 (54) PHYTOCANNABINOIDS IN THE (86). PCT No.: PCT/GB11ASO487 TREATMENT OF CANCER S371 (c)(1), (75) Inventors: Daniela Parolaro, Varese (IT); Paola (2), (4) Date: Nov. 19, 2012 Massi,Izzo, Naples Milan (IT): (IT); Francesca Angelo Antonio Borelli, (30) Foreign ApplicationO O Priority Data Naples (IT): Gabriella Aviello, Naples Mar. 12, 2010 (GB) ................................... 10041374 (IT): Vincenzo DiMarzo, Pozzuoli (IT): Luciano De Petrocellis, Pozzuoli (IT): Publication Classification Aniello Schiano Moriello, Pozzuoli (IT); Alessia Ligresti, Pozzuoli (IT): (51) Int. Cl. Ruth Alexandra Ross, Aberdeen (GB); A61E36/85 (2006.01) Lesley Ann Ford, Aberdeen (GB); A63L/05 (2006.01) Sharon Anavi-Goffer, Aberdeen (GB); A63L/92 (2006.01) Manuel Guzman, Madrid (ES); A6IPI/00 (2006.01) Guillermo Velasco, Madrid (ES); Mar A 6LX3 L/357 (2006.01) Lorente, Madrid (ES); Sofia Torres, A613 L/443 (2006.01) Madrid (ES); Tetsuro Kikuchi, Osaka A613 L/488 (2006.01) (JP); Geoffrey Guy, Wiltshire (GB); A6IP35/00 (2006.01) Colin Stott, Wiltshire (GB); Stephen A613 L/352 (2006.01) Wright, Salisbury (GB); Alan Sutton, A63L/337 (2006.01) Wiltshire (GB); David Potter, Wiltshire (52) U.S. Cl. ........ 424/725; 514/454: 514/729; 514/568; (GB); Etienne De Meijer, Wiltshire 514/733; 514/456; 514/449; 514/463: 514/338; (GB) 514/393 (73) Assignees: OTSUKA PHARMACEUTICAL CO., (57) ABSTRACT LIMITED, Tokyo (JP); GW PHARMA LIMITED, Salisbury (GB) This invention relates to the use of phytocannabinoids, either in an isolated form or in the form of a botanical drug sub (21) Appl. -

INFORMATION to USERS the Most Advanced Technology Has Been Used to Photo Graph and Reproduce This Manuscript from the Microfilm Master
Synthesis of cannabidiol stereoisomers and analogs as potential anticonvulsant agents. Item Type text; Dissertation-Reproduction (electronic) Authors Shah, Vibhakar Jayantilal. Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 02/10/2021 14:16:58 Link to Item http://hdl.handle.net/10150/184523 INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely eve~~t that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginni'ng at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. -

Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma
Supplemental material Cannabigerol is a potential therapeutic agent in a novel combined therapy for glioblastoma Tamara T. Lah, Metka Novak, Milagros A. Pena Almidon, Oliviero Marinelli, Barbara Žvar Baškovič, Bernarda Majc, Mateja Mlinar, Roman Bošnjak, Barbara Breznik, Roby Zomer, and Massimo Nabissi Figure S1. Biosynthesis and metabolism of cannabigerol (CBG). The enzyme prenyltransferase catalyses the conversion of olivetolic acid into CBG in the cannabis plant. CBG is the intermediate biosynthetic precursor of delta‐9‐tetrahydrocannabinol (THC)‐acid and cannabidiol (CBD)‐acid and is converted to THC, CBD, and cannabichromene (CBC), which is then converted into THC, CBD, or CBG by specific synthases (adapted from [30]). Cells 2021, 10, 340. https://doi.org/10.3390/cells10020340 www.mdpi.com/journal/cells Cells 2021, 10, 340 2 of 3 Figure S2. Cell death determination by flow cytometry using Annexin V‐FITC and propidium iodide staining. Cells (U87 and NCH44) were treated with IC50 concentrations of compounds CBG, CBD, and THC for 48 h. Cells were labelled with Annexin V‐FITC and propidium iodide and analysed by flow cytometry. The dot blots represent the results from three biological repeats. Table S1: High‐performance liquid chromatography purity results for the CBG, CBD, and THC solutions used in this study. Cannabinoids Cannabinoids Cannabinoids Concentration Concentration Concentration in CBG in CBD in CBD (mg/ml) (mg/ml) (mg/ml) solution solution solution CBDVA BDL* CBDVA BDL* CBDVA BDL* CBDV BDL* CBDV BDL* CBDV BDL* CBDA BDL* -

Phytocannabinoids
Phytocannabinoids More than 100 structurally and physiologically distinct cannabinoid compounds are unique to plants of the genus Cannabis, known collectively as phytocannabinoids. Cayman Chemical offers authentic reference standards for the most prominent of these phytocannabinoids, as well as their metabolites, from our ISO/IEC 17025 and ISO 17034 certified labs. We are working diligently to introduce many more notable phytocannabinoids. If you cannot find a particular compound of interest in our catalog, please contact us for a custom synthesis estimate. Primary Active Constituents Item No. Product Name Metabolites ISO60156 Cannabidiol (CRM)* Item No. Product Name ISO60158 Δ8-THC (CRM)* 21667 (±)-11-hydroxy-Δ9-THC (CRM)* ISO60157 Δ9-THC (CRM)* 20754 (±)-11-nor-9-carboxy-Δ9-THC (CRM)* *Isotopically labeled standard available *Isotopically labeled standard available Naturally Occurring Acids THC Isomers Item No. Product Name Item No. Product Name 9001573 (±)-Cannabichromenic Acid (dicyclohexylamine salt) 25707 (6aR,9S)-Δ10-THC 18090 Cannabidiolic Acid (CRM) 26528 9(R)-Δ6a,10a-THC 20019 Cannabigerolic Acid (CRM) 26529 9(S)-Δ6a,10a-THC ISO60175 THCA-A (CRM) 26283 Varinolic Acid Discover Our Multi-Component Varinol Series Phytocannabinoid CRM Mixtures Item No. Product Name Available to quantify up to 10 21974 (±)-Cannabichromevarin (CRM) prevalent phytocannabinoids 20165 Cannabidivarin (CRM) Learn more 21664 Cannabivarin on Page 2 18091 Tetrahydrocannabivarin (CRM) of this Other Cannabinoids of Interest brochure Item No. Product Name ISO60163 -

Cbg) White Paper Table of Contents
THE MOTHER OF ALL CANNABINOIDS CANNABIGEROL (CBG) WHITE PAPER TABLE OF CONTENTS 2 CANNABIGEROL (CBG) WHITE PAPER PART 1: PART 5: OVERVIEW / STATEMENT THE ENTOURAGE EFFECT 23 OF PURPOSE 04 Another Mechoulam Discovery 23 Why Cannabigerol? 05 Cannabinoid Ratios 24 Research of Dr. Ethan Russo 25 PART 2: CBG Antifungal Qualities 25 EXECUTIVE SUMMARY 06 Cannabigerol (CBG) 07 PART 6: Two Categories of Cannabinoids 07 CANNABINOIDS 26 CBG: Unique Cannabinoid 08 Isolate vs. Broad-spectrum vs.Full-spectrum 26 Cannabinoid Analogs 27 PART 3: Acidic Precursors 29 ENDOCANNABINOID SYSTEM 09 Varin Analogs 29 Governs Bodily Functions 09 Consumption Avenues & Bioavailability 30 ECS Commonality 10 Relationship to Terpenes 32 Endo vs.Phyto Cannabinoids 10 ECS Evolutionary Path 12 PART 7: ECS Receptor Types & Distribution 13 CANNABIGEROL 33 Agonists & Antagonist Molecules 15 Minor But Common 33 ECS Research Studies 16 Mother of Cannabinoids 34 Receptor Distribution 16 Potential Fourth Chemotype 36 Direct & Indirect Mechanisms 17 CBD: Companion Molecule 38 Safety Profile Investigated 17 Cannabigerivarin (CBGV) 38 GPR18 & GPR55 Receptors 18 Natural Polymorphisms 18 PART 8: More Receptor Distribution Data 19 CBG RESEARCH 39 Appendix: Glossary 45 PART 4: CLINICAL ENDOCANNABINOID DEFICIENCY 21 CED Research Studies 22 The ECS & Depression 22 CANNABIGEROL (CBG) WHITE PAPER 3 The plant species cannabis sativa has been employed by humans PART 1 and wellness practitioners for literally thousands of years—as OVERVIEW / illustrated by recent carbon-dated archeological discoveries cited within this white paper. Several traditional medicinal schools of STATEMENT thought have employed this unique herb (which also qualifies as a vegetable) throughout ancient and modern history. -

Design and Synthesis of Novel Cannabinergic Analogs with Controlled Detoxification
Design and Synthesis of Novel Cannabinergic Analogs with Controlled Detoxification ------------------------------------------------------------------------------ Thesis Presented by Rishi Sharma to The Bouve’ Graduate School of Health Sciences in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Pharmaceutical Sciences with specialization in Medicinal Chemistry and Drug Development NORTHEASTERN UNIVERSITY BOSTON, MASSACHUSETTS April, 2011 Signature page 1 Northeastern University Bouve’ Graduate School of Health Sciences Thesis title: Design and synthesis of novel cannabinergic analogs with controlled detoxification Author: Rishi Sharma Program: Pharmaceutical Sciences with specialization in Medicinal Chemistry and Drug Development Approval for thesis requirement of the Doctor of Philosophy in Pharmaceutical Sciences Thesis Committee (Chairman) ________________________ Date ___________ ________________________ Date ___________ ________________________ Date ___________ ________________________ Date ___________ ________________________ Date ___________ Director of the Graduate School ________________________ Date ___________ Dean ________________________ Date___________ Copy Deposited in Library ________________________ Date___________ Signature page 2 Northeastern University Bouve’ Graduate School of Health Sciences Thesis title: Design and synthesis of novel cannabinergic analogs with controlled detoxification Author: Rishi Sharma Program: Pharmaceutical Sciences with specialization in Medicinal Chemistry