Cannabidiol (CBD) and Its Analogs: a Review of Their Effects on Inflammation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cannabinoid-Induced Hypotension and Bradycardia in Rats Is 1 Mediated by CB1-Like Cannabinoid Receptors
0022-3565/97/2813-1030$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 281, No. 3 Copyright © 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. JPET 281:1030–1037, 1997 Cannabinoid-Induced Hypotension and Bradycardia in Rats Is 1 Mediated by CB1-Like Cannabinoid Receptors KRISTY D. LAKE, DAVID R. COMPTON, KAROLY VARGA, BILLY R. MARTIN and GEORGE KUNOS Department of Pharmacology and Toxicology, Medical College of Virginia, Virginia Commonwealth University, Richmond, Virginia Accepted for publication February 19, 1997 ABSTRACT 9 Previous studies indicate that the CB1 cannabinoid receptor an- potency was (-)-11-OH-D -THC dimethylheptyl $ (-)-3-[2- tagonist, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophe- hydroxy-4-(1,1-dimethyl-heptyl)phenyl]-4-[3-hydroxy-propyl]cy- nyl)-4-methyl-1H-pyrazole-3-carboxamide HCl (SR141716A), in- clohexan-1-ol . (-)-3-[2-hydroxy-4-(1,1-dimethyl-heptyl)phenyl]- hibits the anandamide- and D9-tetrahydrocannabinol- (THC) 4-[3-hydroxy-propyl]cyclohexan-1-ol . THC . anandamide $ induced hypotension and bradycardia in anesthetized rats with a (-)-3-[2-hydroxy-4-(1,1-dimethyl-heptyl)phenyl]-4-[3-hydroxy- potency similar to that observed for SR141716A antagonism of propyl]cyclohexan-1-ol, which correlated well with CB1 receptor THC-induced neurobehavioral effects. To further test the role of affinity or analgesic potency (r 5 0.96-0.99). There was no hypo- CB1 receptors in the cardiovascular effects of cannabinoids, we tension or bradycardia after palmitoylethanolamine or (1)-11-OH- examined two additional criteria for receptor-specific interactions: D9-THC dimethylheptyl. -
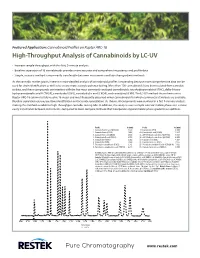
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

Medicinal Chemistry Endeavors Around the Phytocannabinoids
CHEMISTRY & BIODIVERSITY – Vol. 4 (2007) 1707 REVIEW Medicinal Chemistry Endeavors around the Phytocannabinoids by Eric Stern and Didier M. Lambert* Drug Design and Discovery Center and Unite´ de Chimie pharmaceutique et de Radiopharmacie, Ecole de Pharmacie, Faculte´ de Me´decine, Universite´ catholique de Louvain, Avenue E. Mounier 73, U.C.L. 73.40, B-1200 Bruxelles (phone: þ3227647347; fax: þ3227647363; e-mail: [email protected]) Over the past 50 years, a considerable research in medicinal chemistry has been carried out around the natural constituents of Cannabis sativa L. Following the identification of D9-tetrahydrocannabinol (D9-THC) in 1964, critical chemical modifications, e.g., variation of the side chain at C(3) and the opening of the tricyclic scaffold, have led to the characterization of potent and cannabinoid receptor subtype-selective ligands. Those ligands that demonstrate high affinity for the cannabinoid receptors and good biological efficacy are still used as powerful pharmacological tools. This review summarizes past as well as recent developments in the structure–activity relationships of phytocannabinoids. 1. Introduction. – Despite the wide uses of preparations of the hemp Cannabis sativa L. during the History, the modern pharmacology of natural cannabinoids has been hampered by the slow progress in the elucidations of the chemical structures of its major components. Indeed, it is nowadays known that more than 70 compounds derived from a diterpene structure are present in the plant [1], and this fact may explain the difficulty to obtain pure chemical entities in the past. In addition, the medicinal research for more than a half century has been driven by the search for the components responsible for the psychoactive effects of cannabis, this era in the history of the chemical research on cannabinoids have been recently reviewed [2][3]. -

Recent Developments in Cannabis Chemistry
Recent Developments in Cannabis Chemistry BY ALEXANDER T. SHULGIN, Ph.D. The marijuana plant Cannabis sativa contains a bewildering Introduction array of organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono- and sesquiterpenes, carbohy- drates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies. The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitro- gen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of terpene and a substituted resorcinol. Beyond the scope of this present review are such questions as the distribution of these compounds within the plant, the bo- tanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several Reprinted from Journal of Psychedelic Drugs, vol. II, no. 1, 197 1. 397 398 Marijuana: Medical Papers distinct compounds present, and the various preparations and customs of administration. -

Preparative Isolation of Cannabinoids from Cannabis Sativa by Centrifugal Partition Chromatography
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/244594577 Preparative Isolation of Cannabinoids from Cannabis sativa by Centrifugal Partition Chromatography Article in Journal of Liquid Chromatography & Related Technologies · December 2004 DOI: 10.1081/JLC-200028170 CITATIONS READS 47 2,645 5 authors, including: Arno Hazekamp Robert Verpoorte Bedrocan BV Leiden University 50 PUBLICATIONS 975 CITATIONS 732 PUBLICATIONS 21,203 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: The effects of inhaled delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on pain relief and subjective effects in fibromyalgia patients View project All content following this page was uploaded by Robert Verpoorte on 20 June 2014. The user has requested enhancement of the downloaded file. Cannabis; extracting the medicine i Arno Hazekamp Cannabis; extracting the medicine Proefschrift Universiteit Leiden ISBN 978-90-9021997-4 Printed by PrintPartners Ipskamp B.V., Amsterdam, The Netherlands Paper cover: Cannabis Pur 100% (250 grams) from Grafisch Papier, The Nederlands. Photo cover: Dutch medicinal cannabis, variety “Bedrocan”. ii Cannabis; extracting the medicine Proefschrift Ter verkrijging van de graad van Doctor aan de Universiteit Leiden, op gezag van de Rector Magnificus prof. mr. P. F. van der Heijden, hoogleraar in de faculteit der Rechtsgeleerdheid, volgens besluit van het College voor Promoties te verdedigen op woensdag 5 september 2007 klokke 15.00 uur door Arno Hazekamp Geboren op 15 maart 1976 te Bilthoven iii Promotiecommissie Promotor Prof. dr. R. Verpoorte Referent Dr. C. Giroud (Institut Universitaire de Médecine Légale, Lausanne, Switzerland) Overige leden Prof. -

Icrs2015 Programme
TH 25 ANNUAL SYMPOSIUM OF THE INTERNATIONAL CANNABINOID RESEARCH SOCIETY WOLFVILLE NOVA SCOTIA JUNE 28 - JULY 3, 2015 TH 25 ANNUAL SYMPOSIUM OF THE INTERNATIONAL CANNABINOID RESEARCH SOCIETY WOLFVILLE JUNE 28 – JULY 3, 2015 Symposium Programming by Cortical Systematics LLC Copyright © 2015 International Cannabinoid Research Society Research Triangle Park, NC USA ISBN: 978-0-9892885-2-1 These abstracts may be cited in the scientific literature as follows: Author(s), Abstract Title (2015) 25th Annual Symposium on the Cannabinoids, International Cannabinoid Research Society, Research Triangle Park, NC, USA, Page #. Funding for this conference was made possible in part by grant 5R13DA016280-13 from the National Institute on Drug Abuse. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government. Sponsors ICRS Government Sponsors National Institute on Drug Abuse Non- Profit Organization Sponsors Kang Tsou Memorial Fund 2015 ICRS Board of Directors Executive Director Cecilia Hillard, Ph.D. President Stephen Alexander, Ph.D. President- Elect Michelle Glass, Ph.D. Past President Ethan Russo, M.D. Secretary Steve Kinsey, Ph.D. Treasurer Mary Abood, Ph.D. International Secretary Roger Pertwee, D . Phil. , D . S c. Student Representative Tiffany Lee, Ph.D. Grant PI Jenny Wiley, Ph.D. Managing Director Jason Schechter, Ph.D. 2015 Symposium on the Cannabinoids Conference Coordinators Steve Alexander, Ph.D. Cecilia Hillard, Ph.D. Mary Lynch, M.D. Jason Schechter, Ph.D. -

Effects of Abnormal Cannabidiol on Oxytocin-Induced Myometrial Contractility
REPRODUCTIONRESEARCH Effects of abnormal cannabidiol on oxytocin-induced myometrial contractility Diarmaid D Houlihan, Michael C Dennedy and John J Morrison Department of Obstetrics and Gynecology, Clinical Sciences Institute, University College Hospital, National University of Ireland Galway, Newcastle Road, Galway, Ireland Correspondence should be addressed to J J Morrison; Email: [email protected] Abstract The objective of this study was to investigate the effects of abnormal cannabidiol (abn-cbd) on oxytocin-induced myometrial contractility occurring during pregnancy. Isometric tension recordings were performed in isolated myometrial strips from biopsies obtained at K K elective cesarean section. The effects of cumulative doses of abn-cbd (10 9–10 5 M) on oxytocin-induced myometrial contractions alone, and on those following pre-incubation with SR 144528, AM 251, methylene blue, and iberiotoxin were measured, and dose– K response curves were constructed. The pD2 ( log EC50) values and the maximal inhibitory (MMI) values that were achieved were compared for each tissue type. Abn-cbd exerted a potent relaxant effect on oxytocin-induced myometrial contractions in vitro. Pre-incubation with the guanylate cyclase inhibitor, methylene blue, and the BKCa channel antagonist, iberiotoxin, significantly ! ! attenuated this effect (for pD2, P 0.01; for MMI, P 0.01). Abn-cbd exerts a potent inhibitory effect on human uterine contractility. This effect is partially mediated through modulation of guanylate cyclase and activation of BKCa channel activity. These findings have implications for physiologic regulation of myometrial quiescence. Reproduction (2010) 139 783–788 Introduction regioisomer of cannabidiol, which mediates a relaxant effect in isolated rat mesenteric artery via a mechanism The endocannabinoids comprise a family of eicosanoid that is distinct from CB1 and CB2 receptor activation and related unsaturated fatty acid derivatives, of which (Jarai et al. -

Cannabinoids for the Control of Experimental Multiple Sclerosis Pryce, Gareth
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Queen Mary Research Online Cannabinoids for the control of experimental multiple sclerosis Pryce, Gareth The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without the prior written consent of the author For additional information about this publication click this link. https://qmro.qmul.ac.uk/jspui/handle/123456789/673 Information about this research object was correct at the time of download; we occasionally make corrections to records, please therefore check the published record when citing. For more information contact [email protected] 1 Cannabinoids for the control of experimental multiple sclerosis GARETH PRYCE Neuroimmunology Unit, Neuroscience & Trauma Barts and the London School of Medicine and Dentistry Queen Mary University of London UK 2 This thesis is dedicated in memory of my Dad, Glyn Pryce (1927-2010), with love and thanks. 3 ACKNOWLEDGEMENTS I wouldlike to thank DavidBaker andGavin Giovannoni for their supervision and providingme with the opportunity to undertake this thesis. I wouldlike to thank J.Ludovic Croxford, Sam Jackson, Sarah Al-Izki from the lab past and present; Ana Cabranes (RIP) and the Javier Fernadez-Ruiz laboratory in Madrid, Spain; the Vincenzo Di Marzio Laboratory Naples, Italy; the Andy Irving Laboratory, Dundee; the laboratories of Elga De Vries andSandra Amor at the Free University Amsterdam, The Netherlands and the laboratories of Ruth Ross and Roger Pertwee in Aberdeen and Alison Hardcastle University College London for providing me with supportive data and particularly Cristina Visintin and David Selwoodof University College London andCanbex for providingdata from contract Research organizations. -

Expression of Cannabinoid Receptors in Human Osteoarthritic Cartilage
Expression of cannabinoid receptors in human osteoarthritic cartilage: implications for future therapies DUNN, Sara L., WILKINSON, Jeremy Mark, CRAWFORD, Aileen, BUNNING, Rowena A.D. <http://orcid.org/0000-0003-3110-0445> and LE MAITRE, Christine L. <http://orcid.org/0000-0003-4489-7107> Available from Sheffield Hallam University Research Archive (SHURA) at: http://shura.shu.ac.uk/11794/ This document is the author deposited version. You are advised to consult the publisher's version if you wish to cite from it. Published version DUNN, Sara L., WILKINSON, Jeremy Mark, CRAWFORD, Aileen, BUNNING, Rowena A.D. and LE MAITRE, Christine L. (2016). Expression of cannabinoid receptors in human osteoarthritic cartilage: implications for future therapies. Cannabis and Cannabinoid Research, 1 (1), 3-15. Copyright and re-use policy See http://shura.shu.ac.uk/information.html Sheffield Hallam University Research Archive http://shura.shu.ac.uk Cannabis and Cannabinoid Research Volume 1.1, 2016 Cannabis and DOI: 10.1089/can.2015.0001 Cannabinoid Research ORIGINAL RESEARCH Open Access Expression of Cannabinoid Receptors in Human Osteoarthritic Cartilage: Implications for Future Therapies Sara L. Dunn,1 Jeremy Mark Wilkinson,2 Aileen Crawford,3 Rowena A.D. Bunning,1 and Christine L. Le Maitre1,* Abstract Introduction: Cannabinoids have shown to reduce joint damage in animal models of arthritis and reduce ma- trix metalloproteinase expression in primary human osteoarthritic (OA) chondrocytes. The actions of cannabi- noids are mediated by a number of receptors, including cannabinoid receptors 1 and 2 (CB1 and CB2), G- protein-coupled receptors 55 and 18 (GPR55 and GPR18), transient receptor potential vanilloid-1 (TRPV1), and peroxisome proliferator-activated receptors alpha and gamma (PPARa and PPARc). -

The Phytocannabinoides from Cannabis Sativa L. an Overview
Hop and Medicinal Plants, Year XXVIII, No. 1-2, 2020 ISSN 2360–0179 print, ISSN 2360–0187 electronic THE PHYTOCANNABINOIDES FROM CANNABIS SATIVA L. AN OVERVIEW ONA Andreea Daniela, Sorin MUNTEAN, Leon MUNTEAN* University of Agricultural Sciences and Veterinary Medicine, Faculty of Agriculture, Crop Science Department 3-5 Mănăstur Street, 400372, Cluj-Napoca, Romania *Corresponding author e-mail: [email protected] Abstract: Cannabis sativa is among the first cultivated plants for producing fibres from the stalks and consumption of the seeds. Later, the medicinal properties were discovered. The oldest written record of using cannabis for the medicinal and recreational purposes is from China. Over the time many experimental researches were carried out that emphasize the importance of cannabinoids for human health. The present paper focused to present an overview of the main cannabinoids synthetized in cannabis plant and their medicinal and therapeutic effects. Cannabis has a complex chemical composition including besides cannabinoids (over 100), also terpenoids, sugars, alkaloids, quinones and stilbenoids. Of major importance are two compounds tetrahydrocannabinol (THC) and cannabidiol (CBD). Over the time different researches pointed out the beneficial effect of cannabinoids on a wide range of diseases such as epilepsy, neurological diseases, mental conditions like anxiety and depression, chronic pains and certain forms of cancer. In addition to the important advantages it must be also paid attention to the psychoactive action of the certain cannabinoids that may produce cognitive disturbances, attention disorders, sever panic, can affect the short-term memory, and slow the reaction time. This deficits induced usually by THC can be moderate by using CBD which reduce the psychoactive effect. -
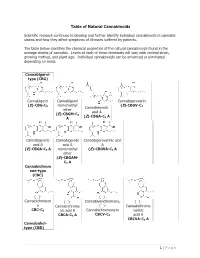
Table of Natural Cannabinoids
Table of Natural Cannabinoids Scientific research continues to develop and further identify individual cannabinoids in cannabis strains and how they affect symptoms of illnesses suffered by patients. The table below identifies the chemical properties of the natural cannabinoids found in the average strains of cannabis. Levels of each of these chemicals will vary with varietal strain, growing method, and plant age. Individual cannabinoids can be enhanced or eliminated depending on need. Cannabigerol- type (CBG) Cannabigerol Cannabigerol Cannabigerovarin (E)-CBG-C monomethyl (E)-CBGV-C 5 Cannabinerolic 3 ether acid A (E)-CBGM-C 5 (Z)-CBGA-C A A 5 Cannabigerolic Cannabigerolic Cannabigerovarinic acid acid A acid A A (E)-CBGA-C5 A monomethyl (E)-CBGVA-C3 A ether (E)-CBGAM- C5 A Cannabichrom ene-type (CBC) (±)- (±)- Cannabichromen (±)- Cannabivarichromene, (±)- e Cannabichrome (±)- Cannabichrome CBC-C5 nic acid A Cannabichromevarin varinic CBCA-C5 A CBCV-C3 acid A CBCVA-C3 A Cannabidiol- type (CBD) 1 | Page (−)-Cannabidiol Cannabidiol Cannabidiol-C4 (−)- Cannabidiorc CBD-C5 momomethyl CBD-C4 Cannabidivarin ol ether CBDV-C3 CBD-C1 CBDM-C5 Cannabidiolic Cannabidivarini acid c acid CBDA-C5 CBDVA-C3 Cannabinodiol- type (CBND) Cannabinodiol Cannabinodivar CBND-C5 in CBND-C3 Tetrahydrocan nabinol-type (THC) 9 9 9 Δ - Δ - Δ - Δ9- Tetrahydrocanna Tetrahydrocan Tetrahydrocannabivarin 9 Tetrahydrocan binol nabinol-C4 Δ -THCV-C3 9 9 nabiorcol Δ -THC-C5 Δ -THC-C4 9 Δ -THCO-C1 9 9 Δ -Tetrahydro- Δ9-Tetrahydro- Δ -Tetrahydro- Δ9-Tetrahydro- cannabinolic -

Cannabinoid Receptors and Endocannabinoids
The AAPS Journal 2006; 8 (2) Article 34 (http://www.aapsj.org). Themed Issue: Lipid Mediator Lipidomics: New Pathways in Mapping Resolution Guest Editors - Charles N. Serhan, Song Hong and Yan Lu Cannabinoid Receptors and Endocannabinoids: Evidence for New Players Submitted: December 29 , 2005 ; Accepted: February 28 , 2006; Published: April 28, 2006 Ken Mackie1 and Nephi Stella2 1 Departments of Anesthesiology and Physiology and Biophysics, University of Washington, Seattle WA 2 Departments of Pharmacology, Psychiatry, and Behavioral Sciences, University of Washington, Seattle, WA A BSTRACT nabinoids, and are getting much closer to overcoming It is now well established that the psychoactive effects of these 2 challenges. Cannabis sativa are primarily mediated through neuronal C sativa contains ~60 phytocannabinoids, a handful of which CB1 receptors, while its therapeutic immune properties are bioactive as defi ned by their ability to specifi cally inter- are primarily mediated through CB2 receptors. Two endo- act with membrane-associated receptors, the cannabinoid cannabinoids, arachidonoylethanolamide and 2-arachi- receptors. The best-known phytocannabinoid is Δ 9 -tetrahy- donoylglycerol, have been identifi ed, their action on CB1 drocannabinol (THC),3 which is thought to mediate most — if and CB2 thoroughly characterized, and their production not all — of the psychotropic and addictive properties of C and inactivation elucidated. However, many signifi cant sativa . 4 Recent evidence suggest that some of the antiinfl am- exceptions to these