Molecular Targets of the Phytocannabinoids-A Complex Picture
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Targeting the Endocannabinoid System to Reduce Nociception
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 Targeting the endocannabinoid system to reduce nociception Lamont Booker Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2419 This Dissertation is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Targeting the Endocannabinoid System to Reduce Nociception A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Virginia Commonwealth University. By Lamont Booker Bachelor’s of Science, Fayetteville State University 2003 Master’s of Toxicology, North Carolina State University 2005 Director: Dr. Aron H. Lichtman, Professor, Pharmacology & Toxicology Virginia Commonwealth University Richmond, Virginia April 2011 Acknowledgements The author wishes to thank several people. I like to thank my advisor Dr. Aron Lichtman for taking a chance and allowing me to work under his guidance. He has been a great influence not only with project and research direction, but as an excellent example of what a mentor should be (always willing to listen, understanding the needs of each student/technician, and willing to provide a hand when available). Additionally, I like to thank all of my committee members (Drs. Galya Abdrakmanova, Francine Cabral, Sandra Welch, Mike Grotewiel) for your patience and willingness to participate as a member. Our term together has truly been memorable! I owe a special thanks to Sheryol Cox, and Dr. -

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -

Role of the Endocannabinoid System and Medical Cannabis
Brigham Young University BYU ScholarsArchive Student Works 2016-12-19 Role of the Endocannabinoid System and Medical Cannabis Sabrina Jarvis Brigham Young University, [email protected] Sean Rasmussen Brigham Young University, [email protected] Blaine Winters Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/studentpub Part of the Nursing Commons The College of Nursing showcases some of our best evidence based scholarly papers from graduate students in the Family Nurse Practitioner Program. The papers address relevant clinical problems for advance practice nurses and are based on the best evidence available. Using a systematic approach students critically analyze and synthesize the research studies to determine the strength of the evidence regarding the clinical problem. Based on the findings, recommendations are made for clinical practice. The papers are published in professional journals and presented at professional meetings. BYU ScholarsArchive Citation Jarvis, Sabrina; Rasmussen, Sean; and Winters, Blaine, "Role of the Endocannabinoid System and Medical Cannabis" (2016). Student Works. 192. https://scholarsarchive.byu.edu/studentpub/192 This Master's Project is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Student Works by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Role of the Endocannabinoid System and Medical Cannabis Sean I. Rasmussen An evidence based scholarly paper submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Masters of Science Sabrina Jarvis, Chair Blaine Winters College of Nursing Brigham Young University Copyright © 2016 Sean I. -
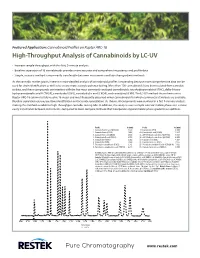
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

Potential Health Benefits of Cannabis Extracts: a Review Maria Rosana Ramirez
International Journal of Chemical and Biomedical Science 2016; 2(1): 1-8 Published online March 1, 2016 (http://www.aascit.org/journal/ijcbs) Potential Health Benefits of Cannabis Extracts: A Review Maria Rosana Ramirez CITER-The National Scientific and Technical Research Council (CONICET), Argentine Government, Agency, Argentina Email address [email protected] Citation Maria Rosana Ramirez. Potential Health Benefits of Cannabis Extracts: A Review. International Journal of Chemical and Biomedical Science . Vol. 2, No. 1, 2016, pp. 1-8. Keywords Abstract Non-cannabinoids Compounds, A central tenet underlying the use of plant preparations is that herbs contain many Cannabis Extracts, bioactive compounds. Cannabis contains tetrahydrocannabinols (THC) a primary Bioactivity metabolite with reported psychotropic effects. Therefore, the presence of THC makes controversial the use of Cannabis to treat diseases by which their uses and applications were limited. The question then is: is it possible to use the extracts from Cannabis to treat the diseases related with it use in folk medicine? More recently, the synergistic Received: January 24, 2016 contributions of bioactive constituents have been scientifically demonstrated. We Revised: February 10, 2016 reviewed the literature concerning medical cannabis and its secondary metabolites, Accepted: February 12, 2016 including fraction and total extracts. Scientific evidence shows that secondary metabolites in cannabis may enhance the positive effects of THC a primary metabolite. Other chemical components (cannabinoid and non-cannabinoid) in cannabis or its extracts may reduce THC-induced anxiety, cholinergic deficits, and immunosuppression; which could increase its therapeutic potential. Particular attention will be placed on non- cannabinoid compounds interactions that could produce synergy with respect to treatment of pain, inflammation, epilepsy, fungal and bacterial infections. -

Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back
International Journal of Molecular Sciences Review Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back Osnat Almogi-Hazan * and Reuven Or Laboratory of Immunotherapy and Bone Marrow Transplantation, Hadassah Medical Center, The Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem 91120, Israel; [email protected] * Correspondence: [email protected] Received: 21 May 2020; Accepted: 19 June 2020; Published: 23 June 2020 Abstract: The Cannabis plant contains numerous components, including cannabinoids and other active molecules. The phyto-cannabinoid activity is mediated by the endocannabinoid system. Cannabinoids affect the nervous system and play significant roles in the regulation of the immune system. While Cannabis is not yet registered as a drug, the potential of cannabinoid-based medicines for the treatment of various conditions has led many countries to authorize their clinical use. However, the data from basic and medical research dedicated to medical Cannabis is currently limited. A variety of pathological conditions involve dysregulation of the immune system. For example, in cancer, immune surveillance and cancer immuno-editing result in immune tolerance. On the other hand, in autoimmune diseases increased immune activity causes tissue damage. Immuno-modulating therapies can regulate the immune system and therefore the immune-regulatory properties of cannabinoids, suggest their use in the therapy of immune related disorders. In this contemporary review, we discuss the roles of the endocannabinoid system in immunity and explore the emerging data about the effects of cannabinoids on the immune response in different pathologies. In addition, we discuss the complexities of using cannabinoid-based treatments in each of these conditions. -

Recent Developments in Cannabis Chemistry
Recent Developments in Cannabis Chemistry BY ALEXANDER T. SHULGIN, Ph.D. The marijuana plant Cannabis sativa contains a bewildering Introduction array of organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono- and sesquiterpenes, carbohy- drates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies. The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitro- gen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of terpene and a substituted resorcinol. Beyond the scope of this present review are such questions as the distribution of these compounds within the plant, the bo- tanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several Reprinted from Journal of Psychedelic Drugs, vol. II, no. 1, 197 1. 397 398 Marijuana: Medical Papers distinct compounds present, and the various preparations and customs of administration. -

What Is Delta-8 THC?? Cannabinoid Chemistry 101
What is Delta-8 THC?? Cannabinoid Chemistry 101 National Conference on Weights and Measures Annual Meeting - Rochester, NY Matthew D. Curran, Ph.D. July 21, 2021 Disclaimer Just to be clear… • I am a chemist and not a lawyer so: • This presentation will not discuss the legal aspects of Δ8-THC or DEA’s current position. • This presentation will not discuss whether Δ8-THC is considered “synthetic” or “naturally occurring.” • This is not a position statement on any issues before the NCWM. • Lastly, this should only be considered a scientific sharing exercise. Florida Department of Agriculture and Consumer Services 2 Cannabis in Florida Cannabis Syllabus • What is Cannabis? • “Mother” Cannabinoid • Decarboxylation • Relationship between CBD and THC • What does “Total” mean? • Dry Weight vs. Wet Weight • What does “Delta-9” mean? • Relationship between “Delta-8” and “Delta-9” • CBD to Delta-8 THC • Cannabinoid Chemistry 202… Florida Department of Agriculture and Consumer Services 3 Cannabis Cannabis • Cannabis sativa is the taxonomic name for the plant. • The concentration of Total Δ9-Tetrahydrocannabinol (Total Δ9-THC) is critical when considering the varieties of Cannabis sativa. • Hemp – (Total Δ9-THC) 0.3% or less • Not really a controversial term, “hemp” • Marijuana/cannabis – (Total Δ9-THC) Greater than 0.3% • Controversial term, “marijuana” • Some states prohibit the use of this term whereas some states have it in their laws. • Some states use the term “cannabis.” • Not italicized • Lower case “c” Florida Department of Agriculture and -

Endocannabinoid System Dysregulation from Acetaminophen Use May Lead to Autism Spectrum Disorder: Could Cannabinoid Treatment Be Efficacious?
molecules Review Endocannabinoid System Dysregulation from Acetaminophen Use May Lead to Autism Spectrum Disorder: Could Cannabinoid Treatment Be Efficacious? Stephen Schultz 1, Georgianna G. Gould 1, Nicola Antonucci 2, Anna Lisa Brigida 3 and Dario Siniscalco 4,* 1 Department of Cellular and Integrative Physiology, Center for Biomedical Neuroscience, University of Texas (UT) Health Science Center San Antonio, San Antonio, TX 78229, USA; [email protected] (S.S.); [email protected] (G.G.G.) 2 Biomedical Centre for Autism Research and Therapy, 70126 Bari, Italy; [email protected] 3 Department of Precision Medicine, University of Campania, 80138 Naples, Italy; [email protected] 4 Department of Experimental Medicine, University of Campania, 80138 Naples, Italy * Correspondence: [email protected] Abstract: Persistent deficits in social communication and interaction, and restricted, repetitive pat- terns of behavior, interests or activities, are the core items characterizing autism spectrum disorder (ASD). Strong inflammation states have been reported to be associated with ASD. The endocannabi- noid system (ECS) may be involved in ASD pathophysiology. This complex network of lipid signal- ing pathways comprises arachidonic acid and 2-arachidonoyl glycerol-derived compounds, their G-protein-coupled receptors (cannabinoid receptors CB1 and CB2) and the associated enzymes. Alter- Citation: Schultz, S.; Gould, G.G.; ations of the ECS have been reported in both the brain and the immune system of ASD subjects. ASD Antonucci, N.; Brigida, A.L.; Siniscalco, D. Endocannabinoid children show low EC tone as indicated by low blood levels of endocannabinoids. Acetaminophen System Dysregulation from use has been reported to be associated with an increased risk of ASD. -

The Cannabinoid WIN 55,212-2 Prevents Neuroendocrine Differentiation of Lncap Prostate Cancer Cells
OPEN Prostate Cancer and Prostatic Diseases (2016) 19, 248–257 www.nature.com/pcan ORIGINAL ARTICLE The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells C Morell1, A Bort1, D Vara2, A Ramos-Torres1, N Rodríguez-Henche1 and I Díaz-Laviada1 BACKGROUND: Neuroendocrine (NE) differentiation represents a common feature of prostate cancer and is associated with accelerated disease progression and poor clinical outcome. Nowadays, there is no treatment for this aggressive form of prostate cancer. The aim of this study was to determine the influence of the cannabinoid WIN 55,212-2 (WIN, a non-selective cannabinoid CB1 and CB2 receptor agonist) on the NE differentiation of prostate cancer cells. METHODS: NE differentiation of prostate cancer LNCaP cells was induced by serum deprivation or by incubation with interleukin-6, for 6 days. Levels of NE markers and signaling proteins were determined by western blotting. Levels of cannabinoid receptors were determined by quantitative PCR. The involvement of signaling cascades was investigated by pharmacological inhibition and small interfering RNA. RESULTS: The differentiated LNCaP cells exhibited neurite outgrowth, and increased the expression of the typical NE markers neuron-specific enolase and βIII tubulin (βIII Tub). Treatment with 3 μM WIN inhibited NK differentiation of LNCaP cells. The cannabinoid WIN downregulated the PI3K/Akt/mTOR signaling pathway, resulting in NE differentiation inhibition. In addition, an activation of AMP-activated protein kinase (AMPK) was observed in WIN-treated cells, which correlated with a decrease in the NE markers expression. Our results also show that during NE differentiation the expression of cannabinoid receptors CB1 and CB2 dramatically decreases. -

Roles of Cannabidiol in Reversing Proteinopathies
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 21 May 2020 doi:10.20944/preprints202005.0340.v1 Review Roles of Cannabidiol in Reversing Proteinopathies Raju Dash1, Md. Chayan Ali2†, Israt Jahan3†, Yeasmin Akter Munni1†, Sarmistha Mitra4, Md. Abdul Hannan1,5, Binod Timalsina1, Diyah Fatimah Oktaviani1, Ho Jin Choi1, Il Soo Moon1* 1Department of Anatomy, Dongguk University College of Medicine, Gyeongju 38066, Republic of Korea 2Department of Biotechnology and Genetic Engineering, Faculty of Biological Sciences, Islamic University, Kushtia 7003, Bangladesh 3Department of Pharmacy, Faculty of Life and Earth Sciences, Jagannath University, Dhaka 1100, Bangladesh. 4Plasma Bioscience Research Center and Department of Plasma Bio display, Kwangwoon University, Seoul 01897, Republic of Korea 5Department of Biochemistry and Molecular Biology, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh †These authors have contributed equally. *Corresponding author: Il Soo Moon Mailing address: Department of Anatomy, Dongguk University College of Medicine, 123 Dongdae-ro, Gyeongju 38066, Republic of Korea Phone: +82-54-770-2414 Fax: +82-54-770-2447 Email: [email protected] Abstract: Cannabidiol is a well-known non-psychotropic phytocannabinoid from Cannabis sativa, which exerts a broad range of neuropharmacological activities in the central nervous systems. Over the past years, compelling evidence from preclinical and clinical studies support therapeutic potentials of cannabidiol in various neurological disorders, including neurodegenerative diseases. Neurodegenerative diseases are characterized by the accumulation of misfolded or aggregated protein due to the defective protein homeostasis or proteostasis network, termed as proteinopathies. Because of its role in the protein homeostasis network, cannabidiol could be a potent molecule to revert not only age-associated neurodegeneration but also other protein misfolding disorders. -

In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa
EDUCATUM JSMT Vol. 4 No. 2 (2017) ISSN 2289-7070 / eISSN 2462-2451 (1-7) https://ejournal.upsi.edu.my/journal/EDSC In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa Shakinaz Desa1*, Asiah Osman2, and Richard Hyslop3 1Department of Biology, Universiti Pendidikan Sultan Idris, Malaysia, 2Natural Product Division, Forest Research Institute Malaysia, 3Department of Chemistry and Biochemistry, University of Northern Colorado, USA *Corresponding author: [email protected] Abstract This study investigated drug-like properties of phytocannabinoids in Cannabis sativa using an in silico study. We report sixteen phytocannabinoids: cannabidiol (CBD), cannabidiolic acid (CBDA), cannabinol (CBN), cannabichromene (CBC), cannabigerol (CBG), cannabicyclol (CBL), cannabivarin (CBV), cannabidivarin (CBDV), cannabichromevarin (CBCV), cannabigerovarin (CBGV), cannabinodiol (CBDL), cannabielsoin (CBE), cannabitriol (CBT), Δ9-tetrahydrocannabinol (Δ9-THC), Δ9-tetrahydrocannabivarin (Δ9-THCV), and Δ8-tetrahydrocannabinol (Δ8-THC). All chemical structures and properties were obtained from PubChem Compound, National Center for Biotechnology Information, U.S. National Library of Medicine. Molinspiration was used for the calculation of molecular properties and bioactivity score. The parameters were molecular weight (MW), number of hydrogen acceptor (HBA), number of hydrogen donor (HBD), partition coefficient (cLogP), polar surface area (PSA) and number of rotatable bonds (NROTB). We predicted bioactivity scores for G Protein-Coupled Receptors (GPCR) ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. Lipinski’s rule was used as reference to determine the drug-like properties of the phytocannabinoids. All compounds have MW<500, HBA<10, HBD<5, TPSA<140Å2 and NRTOB<10. Bioactivity score showed an active or moderately active in all compounds.