Drug Pipeline MONTHLY UPDATE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -
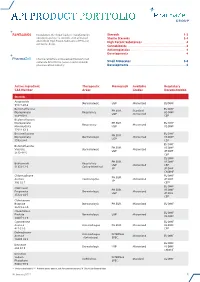
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC. -

Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR)
Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR) October 20, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004-2020 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Manufacturer Indication(s) Corticosteroids – Ophthalmic Topical dexamethasone (Maxidex®)1 Alcon/Novartis . -

Durezol Treats Postoperative Inflammation and Pain This Powerful Corticosteroid Is an Effective New Option for Postsurgical Care
THERAPEUTICS Durezol Treats Postoperative Inflammation and Pain This powerful corticosteroid is an effective new option for postsurgical care. BY MICHAEL KORENFELD, MD cular surgery has undergone an extraordi- nary evolution. Much of the science that we now take for granted—drugs, instruments, O technology, and procedures—was devel- oped only within the past few decades. As recently as 1973, ophthalmologists’ treatment options were far more limited than they are today. There were no micro- incisions, self-sealing wounds, or Nd:YAG lasers. IOLs were an exciting innovation, but phacoemulsification had yet to be invented. In terms of ocular drugs, pred- nisolone acetate had just been approved for the treat- ment of ocular inflammation. Figure 1. The difluprednate molecule. Today, cataract surgery with IOL implantation is one of the most common surgical procedures performed in the tion of critical proteins, and hinder the creation of fur- United States. Its impressively low rate of complications ther inflammatory mediators.1 also makes it one of the safest operations in this country. The most commonly prescribed ophthalmic cortico- Incisions have become incredibly small, thanks to the steroid is prednisolone acetate 1%, a strong steroid, formu- development of foldable IOLs and other technological lated as a suspension (data on file with Sirion Therapeutics, breakthroughs. Inc.). The standard recommended dosing for this drug is Pharmacological developments, however, have not q.i.d. for the treatment of inflammation. Loteprednol kept pace with the tremendous advances in ocular sur- etabonate, 0.5%, another commonly prescribed steroid, is gery. We physicians need a steroid that treats inflamma- also typically dosed q.i.d. -

Contact Dermatitis to Medications and Skin Products
Clinical Reviews in Allergy & Immunology (2019) 56:41–59 https://doi.org/10.1007/s12016-018-8705-0 Contact Dermatitis to Medications and Skin Products Henry L. Nguyen1 & James A. Yiannias2 Published online: 25 August 2018 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract Consumer products and topical medications today contain many allergens that can cause a reaction on the skin known as allergic contact dermatitis. This review looks at various allergens in these products and reports current allergic contact dermatitis incidence and trends in North America, Europe, and Asia. First, medication contact allergy to corticosteroids will be discussed along with its five structural classes (A, B, C, D1, D2) and their steroid test compounds (tixocortol-21-pivalate, triamcinolone acetonide, budesonide, clobetasol-17-propionate, hydrocortisone-17-butyrate). Cross-reactivities between the steroid classes will also be examined. Next, estrogen and testosterone transdermal therapeutic systems, local anesthetic (benzocaine, lidocaine, pramoxine, dyclonine) antihistamines (piperazine, ethanolamine, propylamine, phenothiazine, piperidine, and pyrrolidine), top- ical antibiotics (neomycin, spectinomycin, bacitracin, mupirocin), and sunscreen are evaluated for their potential to cause contact dermatitis and cross-reactivities. Finally, we examine the ingredients in the excipients of these products, such as the formaldehyde releasers (quaternium-15, 2-bromo-2-nitropropane-1,3 diol, diazolidinyl urea, imidazolidinyl urea, DMDM hydantoin), the non- formaldehyde releasers (isothiazolinones, parabens, methyldibromo glutaronitrile, iodopropynyl butylcarbamate, and thimero- sal), fragrance mixes, and Myroxylon pereirae (Balsam of Peru) for contact allergy incidence and prevalence. Furthermore, strategies, recommendations, and two online tools (SkinSAFE and the Contact Allergen Management Program) on how to avoid these allergens in commercial skin care products will be discussed at the end. -

April 1, 2019
BUREAU FOR MEDICAL SERVICES EFFECTIVE WEST VIRGINIA MEDICAID PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA 04/01/2019 This is not an all-inclusive list of available covered drugs and includes only Version 2019.2e managed categories. Refer to cover page for complete list of rules governing this PDL. Prior authorization for a non-preferred agent in any class will be given only if there has been a trial of the preferred brand/generic equivalent or preferred formulation of the active ingredient, at a therapeutic dose, that resulted in a partial response with a documented intolerance. Prior authorization of a non-preferred isomer, pro-drug, or metabolite will be considered with a trial of a preferred parent drug of the same chemical entity, at a therapeutic dose, that resulted in a partial response with documented intolerance or a previous trial and therapy failure, at a therapeutic dose, with a preferred drug of a different chemical entity indicated to treat the submitted diagnosis. (The required trial may be overridden when documented evidence is provided that the use of these preferred agent(s) would be medically contraindicated.) Unless otherwise specified, the listing of a particular brand or generic name includes all legend forms of that drug. OTC drugs are not covered unless specified. PA criteria for non-preferred agents apply in addition to general Drug Utilization Review policy that is in effect for the entire pharmacy program, including, but not limited to, appropriate dosing, duplication of therapy, etc. The use of pharmaceutical samples will not be considered when evaluating the members’ medical condition or prior prescription history for drugs that require prior authorization. -

Prior Authorization PDL Implementation Schedule
Prior Authorization PDL Implementation Schedule Effective Date: Item January 1, 2017 – Descriptive Therapeutic Class Drugs on PDL Drugs which Require PA Nbr Updated: March 1, 2018 1 ADD/ADHD Amphetamine Salt Combo Tablet ( Generic Adderall®) Amphetamine ODT (Adzenys® XR ODT) Stimulants and Related Agents Amphetamine Salt Combo ER (Adderall XR®) Amphetamine Suspension (Dyanavel XR®) Atomoxetine Capsule (Strattera®) Amphetamine Salt Combo ER (Generic; Authorized Generic for Adderall XR) Dexmethylphenidate (Generic; Authorized Generic of Focalin®) Amphetamine Sulfate Tablet (Evekeo®) Dexmethylphenidate ER (Focalin XR®) Armodafinil Tablet (Generic; Authorized Generic; Nuvigil®) Dextroamphetamine Solution (Procentra®) Clonidine ER Tablet (Generic; Kapvay®) Dextroamphetamine Sulfate Tablet (Generic) Dexmethylphenidate (Focalin®) Guanfacine ER Tablet (Generic) Dexmethylphenidate XR (Generic; Authorized Generic for Focalin XR) Lisdexamfetamine Capsule (Vyvanse®) Dextroamphetamine Sulfate Capsule ER (Generic; Dexedrine®Spansule) Methylphenidate IR (Generic) Dextroamphetamine IR Tablet (Zenzedi®) Methylphenidate ER Chew (Quillichew ER®) Dextroamphetamine Solution (Generic for Procentra®) Methylphenidate ER Capsule (Metadate CD®) Guanfacine ER Tablet (Intuniv®) Methylphenidate ER Tablet (Generic; Generic Concerta®; Methamphetamine (Generic; Desoxyn®) Authorized Generic Concerta®) Methylphenidate ER Susp (Quillivant XR®) Methylphenidate IR (Ritalin®) Modafinil Tablet (Generic) Methylphenidate Solution (Generic; Authorized Generic; Methylin®) Methylphenidate -

Federal Bureau of Prisons Health Services National Formulary Part I
Federal Bureau of Prisons Health Services National Formulary Part I Approved: Jeffery D. Allen, MD, MEDICAL DIRECTOR Page 1 of 46 Table of Contents Summary of Formulary Changes Winter 2019 Meeting ..................................................................................................................... 3 National BOP Formulary Mission / Procedural Statement ............................................................................................................................ 4 Definitions/Rules ............................................................................................................................................................................... 6 FDA Medication Guides and Side Effects Statement .......................................................................................................................... 7 Over The Counter Medications .......................................................................................................................................................... 7 Directly Observed Therapy (Formerly “Pill Line”) Only ....................................................................................................................... 7 Non-Substitutable Products ............................................................................................................................................................... 8 Look Alike/Sound Alike Medications ................................................................................................................................................. -

Uveitis Therapy: the Corticosteroid Options
Drugs https://doi.org/10.1007/s40265-020-01314-y REVIEW ARTICLE Uveitis Therapy: The Corticosteroid Options Lianna M. Valdes1 · Lucia Sobrin1 © Springer Nature Switzerland AG 2020 Abstract Uveitis is characterized by intraocular infammation involving the uveal tract; its etiologies generally fall into two broad categories: autoimmune/infammatory or infectious. Corticosteroids are a powerful and important class of medications ubiquitous in the treatment of uveitis. They may be given systemically or locally, in the form of topical drops, periocular injection, intravitreal suspension, or intravitreal implant. This review describes each of the currently available corticosteroid treatment options for uveitis, including favorable and unfavorable characteristics of each as well as applicable clinical trials. The main advantage of corticosteroids as a whole is their ability to quickly and efectively control infammation early on in the course of uveitis. However, they can have serious side efects, whether localized to the eye (such as cataract and elevated intraocular pressure) or systemic (such as osteonecrosis and adrenal insufciency) and in the majority of cases of uveitis are not an appropriate option for long-term therapy. Key Points Clinical features of uveitis include keratic precipitates, ante- rior chamber cell and fare, anterior and posterior synechiae, Corticosteroids are an important mainstay in the treat- iris nodules, snowballs, snowbanks, vitreous haze and cells, ment of uveitis. choroidal lesions, and choroidal thickening. Uncontrolled uveitis can be complicated by cystoid macular edema, retinal Corticosteroids can be given systemically or locally, in vasculitis, optic nerve head edema, and subretinal fuid. A the form of topical drops, periocular injection, intravit- mainstay of treatment for uveitis and its sequelae is the use real suspension, or intravitreal implant; each option has of corticosteroids. -

Therapeutic Drug Class
EFFECTIVE Version Department of Vermont Health Access Updated: 06/05/20 Pharmacy Benefit Management Program /2016 Vermont Preferred Drug List and Drugs Requiring Prior Authorization (includes clinical criteria) The Commissioner for Office of Vermont Health Access shall establish a pharmacy best practices and cost control program designed to reduce the cost of providing prescription drugs, while maintaining high quality in prescription drug therapies. The program shall include: "A preferred list of covered prescription drugs that identifies preferred choices within therapeutic classes for particular diseases and conditions, including generic alternatives" From Act 127 passed in 2002 The following pages contain: • The therapeutic classes of drugs subject to the Preferred Drug List, the drugs within those categories and the criteria required for Prior Authorization (P.A.) of non-preferred drugs in those categories. • The therapeutic classes of drugs which have clinical criteria for Prior Authorization may or may not be subject to a preferred agent. • Within both categories there may be drugs or even drug classes that are subject to Quantity Limit Parameters. Therapeutic class criteria are listed alphabetically. Within each category the Preferred Drugs are noted in the left-hand columns. Representative non- preferred agents have been included and are listed in the right-hand column. Any drug not listed as preferred in any of the included categories requires Prior Authorization. Approval of non-preferred brand name products may require trial and failure of at least 2 different generic manufacturers. GHS/Change Healthcare Change Healthcare GHS/Change Healthcare Sr. Account Manager: PRESCRIBER Call Center: PHARMACY Call Center: Michael Ouellette, RPh PA Requests PA Requests Tel: 802-922-9614 Tel: 1-844-679-5363; Fax: 1-844-679-5366 Tel: 1-844-679-5362 Fax: Note: Fax requests are responded to within 24 hrs. -

Wo 2008/127291 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 23 October 2008 (23.10.2008) WO 2008/127291 A2 (51) International Patent Classification: Jeffrey, J. [US/US]; 106 Glenview Drive, Los Alamos, GOlN 33/53 (2006.01) GOlN 33/68 (2006.01) NM 87544 (US). HARRIS, Michael, N. [US/US]; 295 GOlN 21/76 (2006.01) GOlN 23/223 (2006.01) Kilby Avenue, Los Alamos, NM 87544 (US). BURRELL, Anthony, K. [NZ/US]; 2431 Canyon Glen, Los Alamos, (21) International Application Number: NM 87544 (US). PCT/US2007/021888 (74) Agents: COTTRELL, Bruce, H. et al.; Los Alamos (22) International Filing Date: 10 October 2007 (10.10.2007) National Laboratory, LGTP, MS A187, Los Alamos, NM 87545 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY,BZ, CA, CH, (30) Priority Data: CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, 60/850,594 10 October 2006 (10.10.2006) US ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, (71) Applicants (for all designated States except US): LOS LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, ALAMOS NATIONAL SECURITY,LLC [US/US]; Los MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, Alamos National Laboratory, Lc/ip, Ms A187, Los Alamos, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, NM 87545 (US).