Treatment Options for Ocular Inflammation Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Fluorometholone Ophthalmic Suspension 0.1% W/V Corticosteroid Anti-Inflammatory
PRODUCT MONOGRAPH PrFML® Fluorometholone Ophthalmic Suspension 0.1% w/v Corticosteroid Anti-Inflammatory Allergan Inc. Date of Preparation: Markham, ON October 30, 1972 L6G 0B5 Date of Revision: May 2, 2018 Submission Control No: 214474 Page 1 of 12 NAME OF DRUG Pr ® FML Fluorometholone Ophthalmic Suspension 0.1% w/v THERAPEUTIC CLASSIFICATION Topical corticosteroid ACTIONS Corticosteroids inhibit the inflammatory response to a variety of inciting agents of a mechanical, chemical and immunological nature. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, phagocytic activity, capillary proliferation, fibroblast proliferation, deposition of collagen and scar formation associated with inflammation. Corticosteroids are thought to act by controlling the rate of synthesis of proteins. Corticosteroids and their derivatives are capable of producing a rise in intraocular pressure. INDICATIONS FML® (fluorometholone ophthalmic suspension 0.1% w/v) is indicated for the treatment of steroid- responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe. CONTRAINDICATIONS FML® is contraindicated in: Superficial (or epithelial) herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and other viral diseases of the cornea and conjunctiva. Fungal diseases of ocular structures. Mycobacterial infections of the eye (e.g., Tuberculosis of the eye). Acute untreated infections of the eye. Hypersensitivity to the constituents of this medication (for a listing of ingredients, see PHARMACEUTICAL INFORMATION), or hypersensitivity to other corticosteroids. Page 2 of 12 WARNINGS Use of topical corticosteroids may cause increased intraocular pressure (IOP) in certain individuals. It is necessary that the IOP be checked frequently in patients with a history of glaucoma. Use of corticosteroids may prolong the course and may exacerbate the severity of many viral eye infections (including herpes simplex). -

Docetaxel with Prednisone Or Prednisolone for the Treatment of Prostate Cancer ISSN 1366-5278 Feedback Your Views About This Report
Health Technology Assessment Health Technology Health Technology Assessment 2007; Vol. 11: No. 2 2007; 11: No. 2 Vol. Docetaxel with prednisone or prednisolone for the treatment of prostate cancer A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer Feedback The HTA Programme and the authors would like to know R Collins, E Fenwick, R Trowman, R Perard, your views about this report. The Correspondence Page on the HTA website G Norman, K Light, A Birtle, S Palmer (http://www.hta.ac.uk) is a convenient way to publish and R Riemsma your comments. If you prefer, you can send your comments to the address below, telling us whether you would like us to transfer them to the website. We look forward to hearing from you. January 2007 The National Coordinating Centre for Health Technology Assessment, Mailpoint 728, Boldrewood, Health Technology Assessment University of Southampton, NHS R&D HTA Programme Southampton, SO16 7PX, UK. HTA Fax: +44 (0) 23 8059 5639 Email: [email protected] www.hta.ac.uk http://www.hta.ac.uk ISSN 1366-5278 HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. -
Pharmacy Phacts in This Issue Pharmd Candidates Discuss Oral Health and Preventing Eye Strain at Work
Pharmacy Phacts In this issue PharmD candidates discuss Oral Health and Preventing Eye Strain At Work Oral Health Landon Forrest Stewart, PharmD Candidate 2021 Why is Oral Health important? Oral health is an important part of our overall health. Oral health issues can cause oral pain, increased costs in healthcare, and less productivity. Recent developments in oral hygiene have led to improved outcomes for patients. Many oral health issues are still prevalent today, but the good news is that many are preventable with daily healthy habits. Healthy habits are required to maintain good oral health and many oral health issues are still prevalent today. One of the most common oral health problems is decay in the tooth also known as a cavity. The outer layer of the tooth is a tough mineral layer called the enamel. Bacteria group together on teeth to form plaques that can erode the enamel and the deeper layers of your teeth, causing cavities. Cavities are present and untreated in up to 26% up American adults. (1) If left untreated, they can lead to pain in the tooth and more severe infections known as an abscess. Another common oral health problem is gum disease. When bacteria group together on the teeth, it causes the immune system to respond and causes inflammation in the mouth. The initial inflammation is called gingivitis and makes your gums swell and bleed more easily. Untreated gingivitis can progress to a more severe gum disease called periodontitis. Periodontitis can damage the structure that holds your teeth in place and is a common cause of tooth loss. -

Pre Feasibility Report for Manufacturing of Apis
Pre Feasibility Report For Manufacturing of APIs Cipla Limited Plot No. M12 & M14, Misc. Zone, Phase II, Sector III, Indore SEZ, Pithampur, District Dhar (M.P). - 454775 1 1. IDENTIFICATION OF PROJECT AND PROJECT PROPONENT: CIPLA Ltd is established in the year 1935 as a listed Public Limited Company. CIPLA is engaged in manufacturing of Bulk Drugs and Formulations of wide range of products in the form of tablets, injections, inhalers, capsules, ointment, powder, topical preparations, liquid, syrup drops, sprays, gels, suppositories etc. The company is having its registered office at Mumbai Central, Mumbai – 400 008. 80 years later, the company has become a front runner in the pharmaceutical industry, wielding the latest technology to combat disease and suffering in many ways, touching the lives of thousands the world over.The company’s products are manufactured in 25 state-of- art units. The company is having its manufacturing facilities at following locations: Locations Product Category Vikhroli- Mumbai R&D Virgonagar (Bangalore) Bulk Drugs and Formulations Bommasandra (Bangalore) Bulk Drug Patalganga Bulk Drugs and Formulations Kurkumbh Bulk Drugs and Formulations Goa Formulations Baddi Formulations Sikkim Formulations Indore Formulations The company is having well defined board of directors followed by managerial and technical team looking after entire operation. Management Council- 1. Mr. Umang Vohra - Managing Director and Global Chief Executive Officer 2. Mr. PrabirJha - Global Chief People Officer 3. Mr. KedarUpadhye - Global Chief Financial Officer 4. Dr. Ranjana Pathak - Global Head – Quality 5. Geena Malhotra - Global Head - Integrated Product Development 6. Mr. Raju Subramanyam – Global Head - Operations List of Key Executives- 1. -

Prednisolone Versus Dexamethasone for Croup: a Randomized Controlled Trial Colin M
Prednisolone Versus Dexamethasone for Croup: a Randomized Controlled Trial Colin M. Parker, MBChB, DCH, MRCPCH, FACEM,a,b Matthew N. Cooper, BCA, BSc, PhDc OBJECTIVES: The use of either prednisolone or low-dose dexamethasone in the treatment of abstract childhood croup lacks a rigorous evidence base despite widespread use. In this study, we compare dexamethasone at 0.6 mg/kg with both low-dose dexamethasone at 0.15 mg/kg and prednisolone at 1 mg/kg. METHODS: Prospective, double-blind, noninferiority randomized controlled trial based in 1 tertiary pediatric emergency department and 1 urban district emergency department in Perth, Western Australia. Inclusions were age .6 months, maximum weight 20 kg, contactable by telephone, and English-speaking caregivers. Exclusion criteria were known prednisolone or dexamethasone allergy, immunosuppressive disease or treatment, steroid therapy or enrollment in the study within the previous 14 days, and a high clinical suspicion of an alternative diagnosis. A total of 1252 participants were enrolled and randomly assigned to receive dexamethasone (0.6 mg/kg; n = 410), low-dose dexamethasone (0.15 mg/kg; n = 410), or prednisolone (1 mg/kg; n = 411). Primary outcome measures included Westley Croup Score 1-hour after treatment and unscheduled medical re-attendance during the 7 days after treatment. RESULTS: Mean Westley Croup Score at baseline was 1.4 for dexamethasone, 1.5 for low-dose dexamethasone, and 1.5 for prednisolone. Adjusted difference in scores at 1 hour, compared with dexamethasone, was 0.03 (95% confidence interval 20.09 to 0.15) for low-dose dexamethasone and 0.05 (95% confidence interval 20.07 to 0.17) for prednisolone. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Penetration of Synthetic Corticosteroids Into Human Aqueous Humour
Eye (1990) 4, 526--530 Penetration of Synthetic Corticosteroids into Human Aqueous Humour C. N. 1. McGHEE,1.3 D. G. WATSON, 3 1. M. MIDGLEY, 3 M. 1. NOBLE, 2 G. N. DUTTON, z A. I. FERNl Glasgow Summary The penetration of prednisolone acetate (1%) and fluorometholone alcohol (0.1%) into human aqueous humour following topical application was determined using the very sensitive and specific technique of Gas Chromatography with Mass Spec trometry (GCMS). Prednisolone acetate afforded peak mean concentrations of 669.9 ng/ml within two hours and levels of 28.6 ng/ml in aqueous humour were detected almost 24 hours post application. The peak aqueous humour level of flu orometholone was S.lng/ml. The results are compared and contrasted with the absorption of dexamethasone alcohol (0.1%), betamethasone sodium phosphate (0.1 %) and prednisolone sodium phosphate (0.5%) into human aqueous humour. Topical corticosteroid preparations have been prednisolone acetate (1.0%) and fluorometh used widely in ophthalmology since the early alone alcohol (0.1 %) (preliminary results) 1960s and over the last 10 years the choice of into the aqueous humour of patients under preparations has become larger and more going elective cataract surgery. varied. Unfortunately, data on the intraocular penetration of these steroids in humans has SUbjects and Methods not paralleled the expansion in the number of Patients who were scheduled to undergo rou available preparations; indeed until recently, tine cataract surgery were recruited to the estimation of intraocular penetration has study and informed consent was obtained in been reliant upon extrapolation of data from all cases (n=88), Patients with corneal disease animal models (see Watson et ai., 1988, for or inflammatory ocular conditions which bibliography). -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -

Study Protocol
RESEARCH PROTOCOL Project Title A multicenter, single blind, randomized controlled trial of virucidal effect of Polyvinylpyrrolidone-Iodine on SARS-CoV-2 as well as safety of its application on nasopharynx & oropharynx of COVID-19 positive patients BMRC Reg. No: 38624012021 Page-1/17 Project Title A multicenter, single blind, randomized controlled trial of virucidal effect of Polyvinylpyrrolidone- Iodine on SARS-CoV-2 as well as safety of its application on nasopharynx & oropharynx of COVID- 19 positive patients. Summary Povidone Iodine (Iodine with water soluble polymer Polyvinylpyrolidone) or PVP-I is a proven and time trusted antiseptic agent having best possible (99.99%) virucidal effect in it‟s only 0.23% concentration, against all viruses including SARS-Co, MERS-CoV; even in SARS-COV-2 due to it‟s nonspecific mode of action for virus killing and having no resistance [1,2]. Corona virus is transmitted by/via respiratory droplets or aerosol, produced from sneezing or coughing of infected persons to healthy individual through mouth and nose mainly [5, 6]. The routes of entry of coronavirus in human body are mouth, nose and eye. PVP-I products for gargling the throat and spraying or washing the nose may have a preventive effect on COVID-19 and if it is proved in this study following human trial, this will be a landmark research in COVID-19 pandemic. In line of this, PVP-I containing oro-nasal spray, proposed Bangasafe, which should be regarded as PONS (Povidone Iodine oro-nasal spray) in this protocol, has been developed and proposed to use against corona virus disease. -
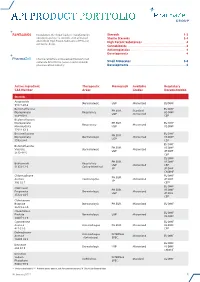
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC.