(CD-P-PH/PHO) Report Classification/Justifica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

DESONATE® (Desonide) Gel 0.05% for Topical Use Only • Systemic Absorption May Require Evaluation for HPA Axis Suppression Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------- WARNINGS AND PRECAUTIONS------------------------ These highlights do not include all the information needed to use • Topical corticosteroids can produce reversible hypothalamic pituitary DESONATE Gel safely and effectively. See full prescribing information adrenal (HPA) axis suppression, Cushing's syndrome and unmask latent for DESONATE Gel. diabetes. (5.1) DESONATE® (desonide) Gel 0.05% for topical use only • Systemic absorption may require evaluation for HPA axis suppression Initial U.S. Approval: 1972 (5.1). • Modify use should HPA axis suppression develop (5.1) ---------------------------- INDICATIONS AND USAGE--------------------------- • Potent corticosteroids, use on large areas, prolonged use or occlusive use may increase systemic absorption (5.1) DESONATE is a corticosteroid indicated for the topical treatment of mild to moderate atopic dermatitis in patients 3 months of age and older. (1) • Local adverse reactions may include atrophy, striae, irritation, acneiform eruptions, hypopigmentation, and allergic contact dermatitis and may be ---------------------- DOSAGE AND ADMINISTRATION----------------------- more likely with occlusive use or more potent corticosteroids. (5.2, 5.4, 6) • Apply as a thin layer to the affected areas two times daily and rub in • Children may be more susceptible to systemic toxicity when treated with gently. (2) topical corticosteroids. (5.1, 8.4) • Therapy should be discontinued when control is achieved. (2) • If no improvement is seen within 4 weeks, reassessment of diagnosis ------------------------------ ADVERSE REACTIONS------------------------------- may be necessary. (2) The most common adverse reactions (incidence ≥ 1%) are headache and • Should not be used with occlusive dressings. (2) application site burning. (6) • Treatment beyond 4 consecutive weeks is not recommended. (2) To report SUSPECTED ADVERSE REACTIONS, contact LEO Pharma • For topical use only. -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -

Clinical Policy: Topical Agents: Corticosteroids
Clinical Policy: Topical Agents: Corticosteroids Reference Number: OH.PHAR.PPA.92 Effective Date: 01/01/2020 Revision Log Last Review Date: Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory and legal information. Description TOPICAL AGENTS: CORTICOSTEROIDS – LOW POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON- PREFERRED” DESONIDE cream, ointment (generic of Desowen®) ALCLOMETASONE cream, ointment (generic of FLUOCINOLONE ACETONIDE 0.01% cream, solution Aclovate®) (generic of Synalar®) CAPEX® shampoo (fluocinolone acetonide) FLUOCINOLONE body oil, scalp oil (generic of Derma- DESONATE®gel (desonide) Smoothe/ FS®) DESONIDE lotion (generic of Desowen®) HYDROCORTISONE cream, lotion, ointment HYDROCORTISONE ACETATE WITH ALOE gel HYDROCORTISONE WITH UREA cream (generic of Carmol HC®) PANDEL® cream (hydrocortisone probutate) PEDIADERM HC® kit TOPICAL AGENTS: CORTICOSTEROIDS – MEDIUM POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON--PREFERRED” BETAMETHASONE DIPROPIONATE-CALCIPOTRIENE BETAMETHASONE DIPROPIONATE lotion (generic of Ointment Diprolene®) BETAMETHASONE VALERATE cream, lotion (generic of CLOCORTOLONE PIVALATE (generic of Cloderm®) Valisone®) CORDRAN® tape (flurandrenolide) FLUTICASONE PROPIONATE cream, ointment (generic of DESOXIMETASONE cream, gel, ointment (generic of Cutivate®) Topicort®) MOMETASONE FUROATE cream, ointment, solution FLUOCINOLONE ACETONIDE 0.025% cream, ointment (generic of Elocon®) (generic of Synalar®) PREDNICARBATE cream (generic of Dermatop®) FLUTICASONE -

Orange Book Cumulative Supplement 7 July 2006
CUMULATIVE SUPPLEMENT 07 July 2006 APPROVED DRUG PRODUCTS WITH THERAPEUTIC EQUIVALENCE EVALUATIONS 26th EDITION Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Generic Drugs 2006 Prepared By Office of Generic Drugs Center for Drug Evaluation and Research Food and Drug Administration APPROVED DRUG PRODUCTS with THERAPEUTIC EQUIVALENCE EVALUATIONS 26th EDITION Cumulative Supplement 07 July 2006 CONTENTS PAGE 1.0 INTRODUCTION ........................................................................................................................................ iii 1.1 How to use the Cumulative Supplement ........................................................................................... iii 1.2 Applicant Name Changes.................................................................................................................. iv 1.3 Availability of the Edition ................................................................................................................... vi 1.4 Report of Counts for the Prescription Drug Product List ................................................................... vi 1.5 Zocor (simvastatin) Patent Relisting.................................................................................................viii 1.6 Cumulative Supplement Legend ....................................................................................................... vi DRUG PRODUCT LISTS Prescription Drug Product List ...................................................................................................... -

Steroids Topical
Steroids, Topical Therapeutic Class Review (TCR) September 18, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. September -

The Management of Common Skin Conditions in General Practice
Management of Common Skin Conditions In General Practice including the “red rash made easy” © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care University of Auckland, Tamaki Campus Reviewed by Hon A/Prof Amanda Oakley - 2019 http://www.dermnetnz.org Management of Common Skin Conditions In General Practice Contents Page Derm Map 3 Classic location: infants & children 4 Classic location: adults 5 Dermatology terminology 6 Common red rashes 7 Other common skin conditions 12 Common viral infections 14 Common bacterial infections 16 Common fungal infections 17 Arthropods 19 Eczema/dermatitis 20 Benign skin lesions 23 Skin cancers 26 Emergency dermatology 28 Clinical diagnosis of melanoma 31 Principles of diagnosis and treatment 32 Principles of treatment of eczema 33 Treatment sequence for psoriasis 34 Topical corticosteroids 35 Combination topical steroid + antimicrobial 36 Safety with topical corticosteroids 36 Emollients 37 Antipruritics 38 For further information, refer to: http://www.dermnetnz.org And http://www.derm-master.com 2 © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care, University of Auckland, Tamaki Campus. Management of Common Skin Conditions In General Practice DERM MAP Start Is the patient sick ? Yes Rash could be an infection or a drug eruption? No Insect Bites – Crop of grouped papules with a central blister or scab. Is the patient in pain or the rash Yes Infection: cellulitis / erysipelas, impetigo, boil is swelling, oozing or crusting? / folliculitis, herpes simplex / zoster. Urticaria – Smooth skin surface with weals that evolve in minutes to hours. No Is the rash in a classic location? Yes See our classic location chart . -
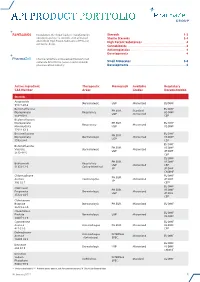
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC. -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

A New Robust Technique for Testing of Glucocorticosteroids in Dogs and Horses Terry E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 A new robust technique for testing of glucocorticosteroids in dogs and horses Terry E. Webster Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Veterinary Toxicology and Pharmacology Commons Recommended Citation Webster, Terry E., "A new robust technique for testing of glucocorticosteroids in dogs and horses" (2007). Retrospective Theses and Dissertations. 15029. https://lib.dr.iastate.edu/rtd/15029 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A new robust technique for testing of glucocorticosteroids in dogs and horses by Terry E. Webster A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Toxicology Program o f Study Committee: Walter G. Hyde, Major Professor Steve Ensley Thomas Isenhart Iowa State University Ames, Iowa 2007 Copyright © Terry Edward Webster, 2007. All rights reserved UMI Number: 1446027 Copyright 2007 by Webster, Terry E. All rights reserved. UMI Microform 1446027 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii DEDICATION I want to dedicate this project to my wife, Jackie, and my children, Shauna, Luke and Jake for their patience and understanding without which this project would not have been possible. -

Medical Review~ Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 21-978 MEDICAL REVIEW~ CLINICAL REVIEW Application Type NDA Submission Number 21-978 Submission Code 000 Letter Date November 18, 2005 Stamp Date November 28, 2005 PDUFA Goal Date September 21,2006 Reviewer Name Denise Cook, M.D. Review Completion Date 8/28/06 Established Name Desonide (Proposed) Trade Name .. Therapeutic Class Topical corticosteroid Applicant Connectics Priority Designatiop S Formulation Foam, 0.05% Dosing Regimen bid Indication F or the treatment of mild to moderate atopic dermatitis . Intended Population Age 3 months and above Clinical Review Denise Cook, M.D. NDA 21-978/ N-OOO _ loam, 0.05%/desonide foam, 0.05% Table of Contents 1 EXECUTIVE SUMMARy................................................................................................................................5 1.1 RECOMMENDATION ON REGULATORY ACTION ...........................................................................................5 1 .2 RECOMMENDATION ON POSTMARKETING ACTIONS. .............. ...... .... .... ........... ........ ..... ... ..... ...... ......... ........5 1.2. I Risk Management Activity................ ......... ..... ............... ..... ................... ........ ........ .... ....... ... ......... ........5 1.2.2 Required Phase 4 Commitments............................................................................................................5 1.2.3 Other Phase 4 Requests..........................................................................................................................5 -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid.