Prior Authorization PDL Implementation Schedule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Betamethasone
Betamethasone Background Betamethasone is a potent, long-acting, synthetic glucocorticoid widely used in equine veterinary medicine as a steroidal anti-inflammatory.1 It is often administered intra-articularly for control of pain associated with inflammation and osteoarthritis.2 Betamethasone is a prescription medication and can only be dispensed from or upon the request of a http://en.wikipedia.org/wiki/Betamethasone#/media/File:Betamethasone veterinarian. It is commercially available .png in a variety of formulations including BetaVet™, BetaVet Soluspan Suspension® and Betasone Aqueous Suspension™.3 Betamethasone can be used intra-articularly, intramuscularly, by inhalation, and topically.4 When administered intra-articularly, it is often combined with other substances such as hyaluronan.5 Intra-articular and intramuscular dosages range widely based upon articular space, medication combination protocol, and practitioner preference. Betamethasone is a glucocorticoid receptor agonist which binds to various glucocorticoid receptors setting off a sequence of events affecting gene transcription and the synthesis of proteins. These mechanisms of action include: • Potential alteration of the G protein-coupled receptors to interfere with intracellular signal transduction pathways • Enhanced transcription in many genes, especially those involving suppression of inflammation. • Inhibition of gene transcription – including those that encode pro-inflammatory substances. The last two of these are considered genomic effects. This type of corticosteroid effect usually occurs within hours to days after administration. The genomic effects persist after the concentrations of the synthetic corticosteroid in plasma are no longer detectable, as evidenced by persistent suppression of the normal production of hydrocortisone following synthetic corticosteroid administration.6 When used judiciously, corticosteroids can be beneficial to the horse. -

Pre Feasibility Report for Manufacturing of Apis
Pre Feasibility Report For Manufacturing of APIs Cipla Limited Plot No. M12 & M14, Misc. Zone, Phase II, Sector III, Indore SEZ, Pithampur, District Dhar (M.P). - 454775 1 1. IDENTIFICATION OF PROJECT AND PROJECT PROPONENT: CIPLA Ltd is established in the year 1935 as a listed Public Limited Company. CIPLA is engaged in manufacturing of Bulk Drugs and Formulations of wide range of products in the form of tablets, injections, inhalers, capsules, ointment, powder, topical preparations, liquid, syrup drops, sprays, gels, suppositories etc. The company is having its registered office at Mumbai Central, Mumbai – 400 008. 80 years later, the company has become a front runner in the pharmaceutical industry, wielding the latest technology to combat disease and suffering in many ways, touching the lives of thousands the world over.The company’s products are manufactured in 25 state-of- art units. The company is having its manufacturing facilities at following locations: Locations Product Category Vikhroli- Mumbai R&D Virgonagar (Bangalore) Bulk Drugs and Formulations Bommasandra (Bangalore) Bulk Drug Patalganga Bulk Drugs and Formulations Kurkumbh Bulk Drugs and Formulations Goa Formulations Baddi Formulations Sikkim Formulations Indore Formulations The company is having well defined board of directors followed by managerial and technical team looking after entire operation. Management Council- 1. Mr. Umang Vohra - Managing Director and Global Chief Executive Officer 2. Mr. PrabirJha - Global Chief People Officer 3. Mr. KedarUpadhye - Global Chief Financial Officer 4. Dr. Ranjana Pathak - Global Head – Quality 5. Geena Malhotra - Global Head - Integrated Product Development 6. Mr. Raju Subramanyam – Global Head - Operations List of Key Executives- 1. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -

Clinical Policy: Topical Agents: Corticosteroids
Clinical Policy: Topical Agents: Corticosteroids Reference Number: OH.PHAR.PPA.92 Effective Date: 01/01/2020 Revision Log Last Review Date: Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory and legal information. Description TOPICAL AGENTS: CORTICOSTEROIDS – LOW POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON- PREFERRED” DESONIDE cream, ointment (generic of Desowen®) ALCLOMETASONE cream, ointment (generic of FLUOCINOLONE ACETONIDE 0.01% cream, solution Aclovate®) (generic of Synalar®) CAPEX® shampoo (fluocinolone acetonide) FLUOCINOLONE body oil, scalp oil (generic of Derma- DESONATE®gel (desonide) Smoothe/ FS®) DESONIDE lotion (generic of Desowen®) HYDROCORTISONE cream, lotion, ointment HYDROCORTISONE ACETATE WITH ALOE gel HYDROCORTISONE WITH UREA cream (generic of Carmol HC®) PANDEL® cream (hydrocortisone probutate) PEDIADERM HC® kit TOPICAL AGENTS: CORTICOSTEROIDS – MEDIUM POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON--PREFERRED” BETAMETHASONE DIPROPIONATE-CALCIPOTRIENE BETAMETHASONE DIPROPIONATE lotion (generic of Ointment Diprolene®) BETAMETHASONE VALERATE cream, lotion (generic of CLOCORTOLONE PIVALATE (generic of Cloderm®) Valisone®) CORDRAN® tape (flurandrenolide) FLUTICASONE PROPIONATE cream, ointment (generic of DESOXIMETASONE cream, gel, ointment (generic of Cutivate®) Topicort®) MOMETASONE FUROATE cream, ointment, solution FLUOCINOLONE ACETONIDE 0.025% cream, ointment (generic of Elocon®) (generic of Synalar®) PREDNICARBATE cream (generic of Dermatop®) FLUTICASONE -

Orange Book Cumulative Supplement 7 July 2006
CUMULATIVE SUPPLEMENT 07 July 2006 APPROVED DRUG PRODUCTS WITH THERAPEUTIC EQUIVALENCE EVALUATIONS 26th EDITION Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Generic Drugs 2006 Prepared By Office of Generic Drugs Center for Drug Evaluation and Research Food and Drug Administration APPROVED DRUG PRODUCTS with THERAPEUTIC EQUIVALENCE EVALUATIONS 26th EDITION Cumulative Supplement 07 July 2006 CONTENTS PAGE 1.0 INTRODUCTION ........................................................................................................................................ iii 1.1 How to use the Cumulative Supplement ........................................................................................... iii 1.2 Applicant Name Changes.................................................................................................................. iv 1.3 Availability of the Edition ................................................................................................................... vi 1.4 Report of Counts for the Prescription Drug Product List ................................................................... vi 1.5 Zocor (simvastatin) Patent Relisting.................................................................................................viii 1.6 Cumulative Supplement Legend ....................................................................................................... vi DRUG PRODUCT LISTS Prescription Drug Product List ...................................................................................................... -

Steroids Topical
Steroids, Topical Therapeutic Class Review (TCR) September 18, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. September -

30 Day Change Notice Effective Date
30 Day Change Notice Effective Date: January 1st, 2021 NEW PREFERRED DRUGS THERAPEUTIC CLASS NO PA REQUIRED PREFERRED Central Nervous System (CNS) Agents: Anticonvulsants Clobazam (Generic of Onfi) Central Nervous System (CNS) Agents: Multiple Aubagio EndocrineSclerosis Agents: Osteoporosis-Bone Ossification Forteo Enhancers Gastrointestinal Agents: Anti-Emetics Bonjesta Genitourinary Agents: Benign Prostatic Hyperplasia Alfuzosin (Generic of Uroxatral) Dutasteride (Generic of Avodart) Genitourinary Agents: Electrolyte Depleter Agents Sevelamer (Generic of Renagel and Renvela) Infectious Disease Agents: Antibiotics-Macrolides Eryped Infectious Disease Agents: Antivirals-HIV Atazanavir Sulfate Oral Powder (Generic of Reyataz) Tivicay PD Infectious Disease Agents: Antibiotics-Tetracyclines Vibramycin Suspension (no PA Required for age 12 or under) Ophthalmic Agents: Antibiotics and Antibiotic -Steroid Neomycin/Polymyxin/Bacitracin/Hydrocortisone Ointment Combination Drops and Ointments Ophthalmic Agents: Glaucoma Agents Dorzolamide/Timolol (Generic of Cosopt PF) NEW CLINICAL PA REQUIRED “PREFERRED” DRUGS THERAPEUTIC CLASS CLINICAL PA REQUIRED PREFERRED Blood Formation, Coagulation, and Thrombosis Agents: Corifact Hemophilia Factors Immunomodulator Agents for Systemic Inflammatory Taltz Disease Immunomodulator Agents for Systemic Inflammatory Xeljanz 5mg Disease NEW STEP THERAPY REQUIRED “PREFERRED” THERAPEUTIC CLASS STEP THERAPY REQUIRED “PREFERRED” Central nervous System (CNS) Agents: Anti-Migraine, Aimovig Prophylaxis Treatment Ajovy -

Comparison of Intramuscular Betamethasone and Oral
Color profile: Disabled Composite Default screen 100 ORIGINAL ARTICLE 100 95 95 75 75 25 25 5 5 0 Comparison of intramuscular 0 betamethasone and oral prednisone in the prevention of relapse of acute asthma John S Chan MD1, Robert L Cowie MD1, Gerald C Lazarenko MD2, Cinde Little RRT1, Sandra Scott RRT1, Gordon T Ford MD FCCP1 1Division of Respiratory Medicine and 2Department of Emergency Medicine, University of Calgary, Calgary, Alberta JS Chan, RL Cowie, GC Lazarenko, C Little, S Scott, tively) and use of inhaled corticosteroids (46% versus 64.3% GT Ford. Comparison of intramuscular betamethasone respectively) (P<0.05). Using intention-to-treat analysis, the and oral prednisone in the prevention of relapse of acute relapse rates for betamethasone and prednisone at day 7 were asthma. Can Respir J 2001;8(3):147-152. 14.9% (13 of 87 patients) and 25% (21 of 84 patients), respectively (P=0.1), and at day 21, the rates were 36.8% (32 OBJECTIVE: To compare the relapse rate after a single intra- of 87 patients) and 31% (26 of 84 patients), respectively muscular injection of a long acting corticosteroid, betametha- (P=0.4). There were no differences in symptom score, peak sone, with oral prednisone in patients discharged from the flows and adverse effects between the two groups at days 7 emergency department (ED) for acute exacerbations of and 21. asthma. CONCLUSIONS: A single dose of intramuscular betametha- PATIENTS AND METHODS: Patients with acute exacer- sone 12 mg was safe and as efficacious as prednisone in pre- bations of asthma who were suitable for discharge from the venting the relapse of acute asthma. -

Long-Term Budesonide Or Nedocromil Mineral Accretion Over a Period of Years
term inhaled corticosteroid (ICS) treatment on bone Long-term Budesonide or Nedocromil mineral accretion over a period of years. Treatment, Once Discontinued, Does Not Alter the Course of Mild to Moderate Asthma in STUDY POPULATION. Cohort follow-up study for a median Children and Adolescents of 7 years with 877 children 5 to 12 years of age who Strunk RC, Sternberg AL, Szefler SJ, et al. J Pediatr. had mild-to-moderate asthma and initially were ran- 2009;154(5):682–687 domly assigned in the Childhood Asthma Management Program. PURPOSE OF THE STUDY. To determine whether long-term, METHODS. Serial dual-energy x-ray absorptiometry scans continuous use of inhaled antiinflammatory medications of the lumbar spine to assess bone mineral density were affects asthma outcomes in children with mild-to-mod- performed for all patients. Annual bone mineral accre- erate asthma after use is discontinued. tion was calculated for 531 boys and 346 girls. STUDY POPULATION. A total of 941 children, 5 to 12 years of RESULTS. Oral corticosteroid bursts produced dose- age, who had previously participated in the Childhood dependent reductions in bone mineral accretion (0.052, Asthma Management Program (CAMP). 0.049, and 0.046 g/cm2 per year with 0, 1–4, and Ն5 courses, respectively) and increases in the risk for osteo- METHODS. During the CAMP trial, subjects received treat- penia (10%, 14%, and 21%, respectively) in boys but ment with budesonide, nedocromil, or placebo for 4.3 not girls. Cumulative ICS use was associated with a small years. During the posttrial period, asthma manage- decrease in bone mineral accretion in boys but not girls ment was provided by primary care physicians ac- but no increased risk for osteopenia. -
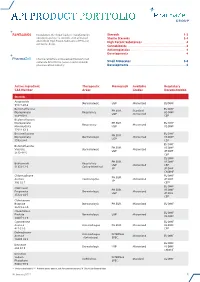
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC. -

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Catalog HPD-5E ® CREATING A HEALTHY WORLDTM Active Pharmaceutical Ingredients (APIs) Available for International Markets Human Pharmaceutical Department www.Pharmapex.net Catalog HPD-5E *Not all products referred to on this site are available in all countries and our products are subject to different regulatory requirements depending on the country of use. Consequently, certain sections of this site may be indicated as being intended only for users in specic countries. Some of the products may also be marketed under different trade names. You should not construe anything on this site as a promotion or solicitation for any product or for the use of any product that is not authorized by the laws and regulations of your country of residence. For inquiries about the availability of any specic product in your country, you may simply contact us at [email protected]. **Products currently covered by valid US Patents may be offered for R&D use in accordance with 35 USC 271(e)+A13(1). Any patent infringement and resulting liability is solely at buyer risk. ©2016, Pharmapex USA, A member of Apex Group of Companies, All Rights Reserved. Toll-Free: 1.844.PHARMAPEX Fax: + 1.619.881.0035 ACTIVE PHARMACEUTICAL [email protected] CREATING A HEALTHY WORLD™ www.Pharmapex.net INGREDIENTS About Pharmapex’s Human Pharmaceuticals Department: Pharmapex’s Human Pharmaceuticals Department (HPD) is a leading source for high-quality Active Pharmaceutical Ingredients (APIs) and Finished Pharmaceutical Products (FPPs) in various markets across the globe. With an extensive product portfolio, our consortium of companies is dedicated to addressing and solving the most important medical needs of our time, including oncology (e.g., multiple myeloma and prostate cancer), neuroscience (e.g., schizophrenia, dementia and pain), infectious disease (e.g., HIV/AIDS, Hepatitis C and tuberculosis), and cardiovascular and metabolic diseases (e.g., diabetes).