2016-01686.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -

Clinical Policy: Topical Agents: Corticosteroids
Clinical Policy: Topical Agents: Corticosteroids Reference Number: OH.PHAR.PPA.92 Effective Date: 01/01/2020 Revision Log Last Review Date: Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory and legal information. Description TOPICAL AGENTS: CORTICOSTEROIDS – LOW POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON- PREFERRED” DESONIDE cream, ointment (generic of Desowen®) ALCLOMETASONE cream, ointment (generic of FLUOCINOLONE ACETONIDE 0.01% cream, solution Aclovate®) (generic of Synalar®) CAPEX® shampoo (fluocinolone acetonide) FLUOCINOLONE body oil, scalp oil (generic of Derma- DESONATE®gel (desonide) Smoothe/ FS®) DESONIDE lotion (generic of Desowen®) HYDROCORTISONE cream, lotion, ointment HYDROCORTISONE ACETATE WITH ALOE gel HYDROCORTISONE WITH UREA cream (generic of Carmol HC®) PANDEL® cream (hydrocortisone probutate) PEDIADERM HC® kit TOPICAL AGENTS: CORTICOSTEROIDS – MEDIUM POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON--PREFERRED” BETAMETHASONE DIPROPIONATE-CALCIPOTRIENE BETAMETHASONE DIPROPIONATE lotion (generic of Ointment Diprolene®) BETAMETHASONE VALERATE cream, lotion (generic of CLOCORTOLONE PIVALATE (generic of Cloderm®) Valisone®) CORDRAN® tape (flurandrenolide) FLUTICASONE PROPIONATE cream, ointment (generic of DESOXIMETASONE cream, gel, ointment (generic of Cutivate®) Topicort®) MOMETASONE FUROATE cream, ointment, solution FLUOCINOLONE ACETONIDE 0.025% cream, ointment (generic of Elocon®) (generic of Synalar®) PREDNICARBATE cream (generic of Dermatop®) FLUTICASONE -

Steroids Topical
Steroids, Topical Therapeutic Class Review (TCR) September 18, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. September -
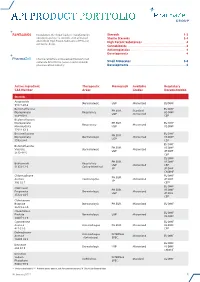
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC. -

Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR)
Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR) October 20, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004-2020 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Manufacturer Indication(s) Corticosteroids – Ophthalmic Topical dexamethasone (Maxidex®)1 Alcon/Novartis . -

Difference Between Patient-Reported Side Effects of Ciclesonide Versus fluticasone Propionate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2010) 104, 1825e1833 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/rmed Difference between patient-reported side effects of ciclesonide versus fluticasone propionate Thys van der Molen a, Juliet M. Foster a,b, Manfred Caeser c,e, Thomas Mu¨ller c, Dirkje S. Postma d,* a Department of General Practice, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands b Woolcock Institute of Medical Research, 431 Glebe Point Rd, Glebe NSW 2037, Sydney, Australia c Nycomed GmbH, Byk-Gulden-Straße 2, 78467 Konstanz, Konstanz, Germany d Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands Received 18 January 2010; accepted 26 May 2010 Available online 2 July 2010 KEYWORDS Summary Adverse events; Rationale: Patient-reported outcomes provide new insights into the dynamics of asthma Inhaled corticosteroid management. Further to asthma control and quality of life, self-reported side effects of treat- questionnaire; ment can be assessed with the validated Inhaled Corticosteroid Questionnaire (ICQ). ICQ; Objectives: To compare patient-reported side effects between the inhaled corticosteroids Patient-reported ciclesonide and fluticasone propionate. outcomes Methods: Patients with moderate or moderate-to-severe asthma, pre-treated with a constant dose and type of medication, were randomized in three separate studies: 1) once daily cicle- sonide 320 mg(n Z 234) or twice daily fluticasone propionate 200 mg(n Z 240); 2) twice daily ciclesonide 320 mg(n Z 255) or twice daily fluticasone propionate 375 mg(n Z 273); and 3) twice daily ciclesonide 320 mg(n Z 259) or twice daily fluticasone propionate 500 mg (n Z 244). -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -

Durezol Treats Postoperative Inflammation and Pain This Powerful Corticosteroid Is an Effective New Option for Postsurgical Care
THERAPEUTICS Durezol Treats Postoperative Inflammation and Pain This powerful corticosteroid is an effective new option for postsurgical care. BY MICHAEL KORENFELD, MD cular surgery has undergone an extraordi- nary evolution. Much of the science that we now take for granted—drugs, instruments, O technology, and procedures—was devel- oped only within the past few decades. As recently as 1973, ophthalmologists’ treatment options were far more limited than they are today. There were no micro- incisions, self-sealing wounds, or Nd:YAG lasers. IOLs were an exciting innovation, but phacoemulsification had yet to be invented. In terms of ocular drugs, pred- nisolone acetate had just been approved for the treat- ment of ocular inflammation. Figure 1. The difluprednate molecule. Today, cataract surgery with IOL implantation is one of the most common surgical procedures performed in the tion of critical proteins, and hinder the creation of fur- United States. Its impressively low rate of complications ther inflammatory mediators.1 also makes it one of the safest operations in this country. The most commonly prescribed ophthalmic cortico- Incisions have become incredibly small, thanks to the steroid is prednisolone acetate 1%, a strong steroid, formu- development of foldable IOLs and other technological lated as a suspension (data on file with Sirion Therapeutics, breakthroughs. Inc.). The standard recommended dosing for this drug is Pharmacological developments, however, have not q.i.d. for the treatment of inflammation. Loteprednol kept pace with the tremendous advances in ocular sur- etabonate, 0.5%, another commonly prescribed steroid, is gery. We physicians need a steroid that treats inflamma- also typically dosed q.i.d. -

Drug Pipeline MONTHLY UPDATE
Drug Pipeline MONTHLY UPDATE Critical updates in an ever changing environment December 2019 NEW DRUG INFORMATION ™ ● Xcopri (cenobamate): The U.S. Food and Drug Administration (FDA) approved SK Biopharmaceuticals’ Xcopri as an antiepileptic drug for treatment of partial-onset seizures in adults. The approval is based on results from two global, randomized, double-blind, placebo-controlled studies and a large, global, multi-center, open-label safety study that enrolled adults with uncontrolled partial-onset seizures, taking one to three concomitant anti-epileptic drug (AEDs). In these studies, Xcopri demonstrated significant reductions in seizure frequency compared to placebo.1 Drug reaction with eosinophilia and systemic symptoms (DRESS) has been reported with Xcopri use. It has not been established that the risk of DRESS is prevented by a slower titration; however, it should be initiated at 12.5 mg once daily and titrated every two weeks. Xcopri is expected to be available in the United States 2Q2020, after scheduling review by the DEA, which typically occurs within 90 days of FDA approval. Price pending. GENERIC DRUG INFORMATION ® + ● Jadenu (deferasirox): Multiple manufacturers have launched their generic version of Novartis’ Jadenu for treatment of chronic iron overload. Jadenu generated $474 million in U.S. annual sales in 2018. ® ● Apriso (mesalamine ER): Multiple manufactures have launched their generic version of Bausch Health’s Apriso for treatment of ulcerative colitis. Apriso generated $312 million in U.S. annual sales in 2018. ® ● Nubupent (pentamidine isethionate): Fresenius launched their generic version of Seton Pharmaceuticals Nebupent inhaled solution for treatment of complicated lung infections. Nubupent does not have any further regulatory exclusivities. -
Ep 2626065 A1
(19) TZZ Z_T (11) EP 2 626 065 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: A61K 31/137 (2006.01) A61K 31/135 (2006.01) 14.08.2013 Bulletin 2013/33 A61K 31/4704 (2006.01) A61K 31/58 (2006.01) A61K 31/56 (2006.01) A61K 9/12 (2006.01) (2006.01) (2006.01) (21) Application number: 11827927.2 A61K 9/14 A61P 11/06 (86) International application number: Date of filing: 01.02.2011 (22) PCT/CN2011/070883 (87) International publication number: WO 2012/041031 (05.04.2012 Gazette 2012/14) (84) Designated Contracting States: (72) Inventor: WU, Wei-hsiu AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Taipei GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Taiwan (TW) PL PT RO RS SE SI SK SM TR (74) Representative: Patentanwaltskanzlei WILHELM (30) Priority: 28.09.2010 CN 201010502339 & BECK Prinzenstrasse 13 (71) Applicant: Intech Biopharm Ltd. 80639 München (DE) Taipei (TW) (54) COMPOUND COMPOSITION FOR INHALATION USED FOR TREATING ASTHMA (57) An inhaled pharmaceutical composition con- tric way as a controller. The eccentric way control therapy tains primary active ingredients of beta2- agonist and cor- could create a low blood concentration period during the ticosteroids.The pharmaceuticalcompositions disclosed day and minimize the acute tolerance phenomenon (or in the present invention are to be inhaled by a patient so called tachyphylaxis) for bronchodilator - beta2-ago- when needed as a reliever, or administrated in an eccen- nists in treating asthma or other obstructive respiratory disorders.