April 1, 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -

Clinical Policy: Topical Agents: Corticosteroids
Clinical Policy: Topical Agents: Corticosteroids Reference Number: OH.PHAR.PPA.92 Effective Date: 01/01/2020 Revision Log Last Review Date: Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory and legal information. Description TOPICAL AGENTS: CORTICOSTEROIDS – LOW POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON- PREFERRED” DESONIDE cream, ointment (generic of Desowen®) ALCLOMETASONE cream, ointment (generic of FLUOCINOLONE ACETONIDE 0.01% cream, solution Aclovate®) (generic of Synalar®) CAPEX® shampoo (fluocinolone acetonide) FLUOCINOLONE body oil, scalp oil (generic of Derma- DESONATE®gel (desonide) Smoothe/ FS®) DESONIDE lotion (generic of Desowen®) HYDROCORTISONE cream, lotion, ointment HYDROCORTISONE ACETATE WITH ALOE gel HYDROCORTISONE WITH UREA cream (generic of Carmol HC®) PANDEL® cream (hydrocortisone probutate) PEDIADERM HC® kit TOPICAL AGENTS: CORTICOSTEROIDS – MEDIUM POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON--PREFERRED” BETAMETHASONE DIPROPIONATE-CALCIPOTRIENE BETAMETHASONE DIPROPIONATE lotion (generic of Ointment Diprolene®) BETAMETHASONE VALERATE cream, lotion (generic of CLOCORTOLONE PIVALATE (generic of Cloderm®) Valisone®) CORDRAN® tape (flurandrenolide) FLUTICASONE PROPIONATE cream, ointment (generic of DESOXIMETASONE cream, gel, ointment (generic of Cutivate®) Topicort®) MOMETASONE FUROATE cream, ointment, solution FLUOCINOLONE ACETONIDE 0.025% cream, ointment (generic of Elocon®) (generic of Synalar®) PREDNICARBATE cream (generic of Dermatop®) FLUTICASONE -

Steroids Topical
Steroids, Topical Therapeutic Class Review (TCR) September 18, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. September -
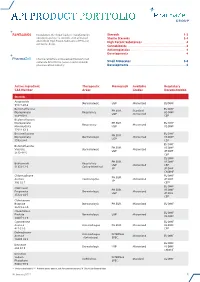
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC. -

Changes to the Highmark Drug Formularies
AUGUST 2020 JULY/AUGUST 2020 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for July/August 2020. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in June 2020 by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Progressive Formulary and the Highmark Progressive Healthcare Reform Formulary C. Changes to the Highmark Healthcare Reform Essential Formulary D. Changes to the Highmark Core Formulary E. Changes to the Highmark National Select Formulary F. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs Section II. Highmark Medicare Part D Formularies A. Changes to the Highmark Medicare Part D 5-Tier Incentive Formulary B. Changes to the Highmark Medicare Part D 5-Tier Closed Formulary C. Additions to the Specialty Tier D. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Quantity Level Limit (QLL) Program As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. -

Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR)
Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR) October 20, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004-2020 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Manufacturer Indication(s) Corticosteroids – Ophthalmic Topical dexamethasone (Maxidex®)1 Alcon/Novartis . -

Durezol Treats Postoperative Inflammation and Pain This Powerful Corticosteroid Is an Effective New Option for Postsurgical Care
THERAPEUTICS Durezol Treats Postoperative Inflammation and Pain This powerful corticosteroid is an effective new option for postsurgical care. BY MICHAEL KORENFELD, MD cular surgery has undergone an extraordi- nary evolution. Much of the science that we now take for granted—drugs, instruments, O technology, and procedures—was devel- oped only within the past few decades. As recently as 1973, ophthalmologists’ treatment options were far more limited than they are today. There were no micro- incisions, self-sealing wounds, or Nd:YAG lasers. IOLs were an exciting innovation, but phacoemulsification had yet to be invented. In terms of ocular drugs, pred- nisolone acetate had just been approved for the treat- ment of ocular inflammation. Figure 1. The difluprednate molecule. Today, cataract surgery with IOL implantation is one of the most common surgical procedures performed in the tion of critical proteins, and hinder the creation of fur- United States. Its impressively low rate of complications ther inflammatory mediators.1 also makes it one of the safest operations in this country. The most commonly prescribed ophthalmic cortico- Incisions have become incredibly small, thanks to the steroid is prednisolone acetate 1%, a strong steroid, formu- development of foldable IOLs and other technological lated as a suspension (data on file with Sirion Therapeutics, breakthroughs. Inc.). The standard recommended dosing for this drug is Pharmacological developments, however, have not q.i.d. for the treatment of inflammation. Loteprednol kept pace with the tremendous advances in ocular sur- etabonate, 0.5%, another commonly prescribed steroid, is gery. We physicians need a steroid that treats inflamma- also typically dosed q.i.d. -

Pharmacy and Poisons Act 1979
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS ACT 1979 1979 : 26 TABLE OF CONTENTS PART I PRELIMINARY 1 Short title 2 Interpretation PART II THE PHARMACY COUNCIL 3 The Pharmacy Council 4 Membership of the Council 4A Functions of the Council 4B Protection from personal liability 4C Annual Report 5 Proceedings of the Council, etc PART III REGISTRATION OF PHARMACISTS 6 Offence to practise pharmacy if not registered 7 Registration as a pharmacist 7A Re-registration as non-practising member 7AA Period of validity of registration 8 Code of Conduct 9 Pharmacy Profession Complaints Committee 10 Investigation of complaint by Committee 10A Inquiry into complaint by Council 10B Inquiry by Council of its own initiative 11 Surrender of registration 12 Restoration of name to register 1 PHARMACY AND POISONS ACT 1979 13 Proof of registration 14 Appeals 14A Fees 14B Amendment of Seventh Schedule 15 Regulations for this part PART IV REGISTRATION OF PHARMACIES 16 Register of pharmacies 17 Registration of premises as registered pharmacies 18 Unfit premises: new applications 19 Unfit premises: registered pharmacies 20 Appeals 21 When certificates of unfitness take effect 22 Regulations for this Part PART V CONTROL OF PRESCRIPTIONS AND IMPORTATION 23 Prescriptions to be in a certain form 23A Validity of a prescription 24 Supply by registered pharmacist of equivalent medicines 25 Restrictions on the importation of medicines 26 Declaration relating to imported medicines [repealed] PART VI CONTROL OF DRUGS 27 Certain substances to be sold on prescription -

Drug Pipeline MONTHLY UPDATE
Drug Pipeline MONTHLY UPDATE Critical updates in an ever changing environment December 2019 NEW DRUG INFORMATION ™ ● Xcopri (cenobamate): The U.S. Food and Drug Administration (FDA) approved SK Biopharmaceuticals’ Xcopri as an antiepileptic drug for treatment of partial-onset seizures in adults. The approval is based on results from two global, randomized, double-blind, placebo-controlled studies and a large, global, multi-center, open-label safety study that enrolled adults with uncontrolled partial-onset seizures, taking one to three concomitant anti-epileptic drug (AEDs). In these studies, Xcopri demonstrated significant reductions in seizure frequency compared to placebo.1 Drug reaction with eosinophilia and systemic symptoms (DRESS) has been reported with Xcopri use. It has not been established that the risk of DRESS is prevented by a slower titration; however, it should be initiated at 12.5 mg once daily and titrated every two weeks. Xcopri is expected to be available in the United States 2Q2020, after scheduling review by the DEA, which typically occurs within 90 days of FDA approval. Price pending. GENERIC DRUG INFORMATION ® + ● Jadenu (deferasirox): Multiple manufacturers have launched their generic version of Novartis’ Jadenu for treatment of chronic iron overload. Jadenu generated $474 million in U.S. annual sales in 2018. ® ● Apriso (mesalamine ER): Multiple manufactures have launched their generic version of Bausch Health’s Apriso for treatment of ulcerative colitis. Apriso generated $312 million in U.S. annual sales in 2018. ® ● Nubupent (pentamidine isethionate): Fresenius launched their generic version of Seton Pharmaceuticals Nebupent inhaled solution for treatment of complicated lung infections. Nubupent does not have any further regulatory exclusivities. -

Phosphodiesterase Inhibitors: Could They Be Beneficial for the Treatment of COVID-19?
International Journal of Molecular Sciences Review Phosphodiesterase Inhibitors: Could They Be Beneficial for the Treatment of COVID-19? Mauro Giorgi 1,*, Silvia Cardarelli 2, Federica Ragusa 3, Michele Saliola 1, Stefano Biagioni 1, Giancarlo Poiana 1 , Fabio Naro 2 and Mara Massimi 3,* 1 Department of Biology and Biotechnology “Charles Darwin”, Sapienza University of Rome, 00185 Rome, Italy; [email protected] (M.S.); [email protected] (S.B.); [email protected] (G.P.) 2 Department of Anatomical, Histological, Forensic Medicine and Orthopedic Sciences, Sapienza University, 00185 Rome, Italy; [email protected] (S.C.); [email protected] (F.N.) 3 Department of Life, Health and Environmental Sciences, University of L’Aquila, 67100 L’Aquila, Italy; [email protected] * Correspondence: [email protected] (M.G.); [email protected] (M.M.) Received: 10 July 2020; Accepted: 24 July 2020; Published: 27 July 2020 Abstract: In March 2020, the World Health Organization declared the severe acute respiratory syndrome corona virus 2 (SARS-CoV2) infection to be a pandemic disease. SARS-CoV2 was first identified in China and, despite the restrictive measures adopted, the epidemic has spread globally, becoming a pandemic in a very short time. Though there is growing knowledge of the SARS-CoV2 infection and its clinical manifestations, an effective cure to limit its acute symptoms and its severe complications has not yet been found. Given the worldwide health and economic emergency issues accompanying this pandemic, there is an absolute urgency to identify effective treatments and reduce the post infection outcomes. In this context, phosphodiesterases (PDEs), evolutionarily conserved cyclic nucleotide (cAMP/cGMP) hydrolyzing enzymes, could emerge as new potential targets. -

PFIZER INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019 ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 1-3619 PFIZER INC. (Exact name of registrant as specified in its charter) Delaware 13-5315170 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification Number) 235 East 42nd Street, New York, New York 10017 (Address of principal executive offices) (zip code) (212) 733-2323 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $.05 par value PFE New York Stock Exchange 0.000% Notes due 2020 PFE20A New York Stock Exchange 0.250% Notes due 2022 PFE22 New York Stock Exchange 1.000% Notes due 2027 PFE27 New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.