Uveitis Therapy: the Corticosteroid Options
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2008/0317805 A1 Mckay Et Al
US 20080317805A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0317805 A1 McKay et al. (43) Pub. Date: Dec. 25, 2008 (54) LOCALLY ADMINISTRATED LOW DOSES Publication Classification OF CORTICOSTEROIDS (51) Int. Cl. A6II 3/566 (2006.01) (76) Inventors: William F. McKay, Memphis, TN A6II 3/56 (2006.01) (US); John Myers Zanella, A6IR 9/00 (2006.01) Cordova, TN (US); Christopher M. A6IP 25/04 (2006.01) Hobot, Tonka Bay, MN (US) (52) U.S. Cl. .......... 424/422:514/169; 514/179; 514/180 (57) ABSTRACT Correspondence Address: This invention provides for using a locally delivered low dose Medtronic Spinal and Biologics of a corticosteroid to treat pain caused by any inflammatory Attn: Noreen Johnson - IP Legal Department disease including sciatica, herniated disc, Stenosis, mylopa 2600 Sofamor Danek Drive thy, low back pain, facet pain, osteoarthritis, rheumatoid Memphis, TN38132 (US) arthritis, osteolysis, tendonitis, carpal tunnel syndrome, or tarsal tunnel syndrome. More specifically, a locally delivered low dose of a corticosteroid can be released into the epidural (21) Appl. No.: 11/765,040 space, perineural space, or the foramenal space at or near the site of a patient's pain by a drug pump or a biodegradable drug (22) Filed: Jun. 19, 2007 depot. E Day 7 8 Day 14 El Day 21 3OO 2OO OO OO Control Dexamethasone DexamethasOne Dexamethasone Fuocinolone Fluocinolone Fuocinolone 2.0 ng/hr 1Ong/hr 50 ng/hr 0.0032ng/hr 0.016 ng/hr 0.08 ng/hr Patent Application Publication Dec. 25, 2008 Sheet 1 of 2 US 2008/0317805 A1 900 ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 80.0 - 7OO – 6OO - 5OO - E Day 7 EDay 14 40.0 - : El Day 21 2OO - OO = OO – Dexamethasone Dexamethasone Dexamethasone Fuocinolone Fluocinolone Fuocinolone 2.0 ng/hr 1Ong/hr 50 ng/hr O.OO32ng/hr O.016 ng/hr 0.08 nghr Patent Application Publication Dec. -

Fluorometholone Ophthalmic Suspension 0.1% W/V Corticosteroid Anti-Inflammatory
PRODUCT MONOGRAPH PrFML® Fluorometholone Ophthalmic Suspension 0.1% w/v Corticosteroid Anti-Inflammatory Allergan Inc. Date of Preparation: Markham, ON October 30, 1972 L6G 0B5 Date of Revision: May 2, 2018 Submission Control No: 214474 Page 1 of 12 NAME OF DRUG Pr ® FML Fluorometholone Ophthalmic Suspension 0.1% w/v THERAPEUTIC CLASSIFICATION Topical corticosteroid ACTIONS Corticosteroids inhibit the inflammatory response to a variety of inciting agents of a mechanical, chemical and immunological nature. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, phagocytic activity, capillary proliferation, fibroblast proliferation, deposition of collagen and scar formation associated with inflammation. Corticosteroids are thought to act by controlling the rate of synthesis of proteins. Corticosteroids and their derivatives are capable of producing a rise in intraocular pressure. INDICATIONS FML® (fluorometholone ophthalmic suspension 0.1% w/v) is indicated for the treatment of steroid- responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe. CONTRAINDICATIONS FML® is contraindicated in: Superficial (or epithelial) herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and other viral diseases of the cornea and conjunctiva. Fungal diseases of ocular structures. Mycobacterial infections of the eye (e.g., Tuberculosis of the eye). Acute untreated infections of the eye. Hypersensitivity to the constituents of this medication (for a listing of ingredients, see PHARMACEUTICAL INFORMATION), or hypersensitivity to other corticosteroids. Page 2 of 12 WARNINGS Use of topical corticosteroids may cause increased intraocular pressure (IOP) in certain individuals. It is necessary that the IOP be checked frequently in patients with a history of glaucoma. Use of corticosteroids may prolong the course and may exacerbate the severity of many viral eye infections (including herpes simplex). -

Pre Feasibility Report for Manufacturing of Apis
Pre Feasibility Report For Manufacturing of APIs Cipla Limited Plot No. M12 & M14, Misc. Zone, Phase II, Sector III, Indore SEZ, Pithampur, District Dhar (M.P). - 454775 1 1. IDENTIFICATION OF PROJECT AND PROJECT PROPONENT: CIPLA Ltd is established in the year 1935 as a listed Public Limited Company. CIPLA is engaged in manufacturing of Bulk Drugs and Formulations of wide range of products in the form of tablets, injections, inhalers, capsules, ointment, powder, topical preparations, liquid, syrup drops, sprays, gels, suppositories etc. The company is having its registered office at Mumbai Central, Mumbai – 400 008. 80 years later, the company has become a front runner in the pharmaceutical industry, wielding the latest technology to combat disease and suffering in many ways, touching the lives of thousands the world over.The company’s products are manufactured in 25 state-of- art units. The company is having its manufacturing facilities at following locations: Locations Product Category Vikhroli- Mumbai R&D Virgonagar (Bangalore) Bulk Drugs and Formulations Bommasandra (Bangalore) Bulk Drug Patalganga Bulk Drugs and Formulations Kurkumbh Bulk Drugs and Formulations Goa Formulations Baddi Formulations Sikkim Formulations Indore Formulations The company is having well defined board of directors followed by managerial and technical team looking after entire operation. Management Council- 1. Mr. Umang Vohra - Managing Director and Global Chief Executive Officer 2. Mr. PrabirJha - Global Chief People Officer 3. Mr. KedarUpadhye - Global Chief Financial Officer 4. Dr. Ranjana Pathak - Global Head – Quality 5. Geena Malhotra - Global Head - Integrated Product Development 6. Mr. Raju Subramanyam – Global Head - Operations List of Key Executives- 1. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Penetration of Synthetic Corticosteroids Into Human Aqueous Humour
Eye (1990) 4, 526--530 Penetration of Synthetic Corticosteroids into Human Aqueous Humour C. N. 1. McGHEE,1.3 D. G. WATSON, 3 1. M. MIDGLEY, 3 M. 1. NOBLE, 2 G. N. DUTTON, z A. I. FERNl Glasgow Summary The penetration of prednisolone acetate (1%) and fluorometholone alcohol (0.1%) into human aqueous humour following topical application was determined using the very sensitive and specific technique of Gas Chromatography with Mass Spec trometry (GCMS). Prednisolone acetate afforded peak mean concentrations of 669.9 ng/ml within two hours and levels of 28.6 ng/ml in aqueous humour were detected almost 24 hours post application. The peak aqueous humour level of flu orometholone was S.lng/ml. The results are compared and contrasted with the absorption of dexamethasone alcohol (0.1%), betamethasone sodium phosphate (0.1 %) and prednisolone sodium phosphate (0.5%) into human aqueous humour. Topical corticosteroid preparations have been prednisolone acetate (1.0%) and fluorometh used widely in ophthalmology since the early alone alcohol (0.1 %) (preliminary results) 1960s and over the last 10 years the choice of into the aqueous humour of patients under preparations has become larger and more going elective cataract surgery. varied. Unfortunately, data on the intraocular penetration of these steroids in humans has SUbjects and Methods not paralleled the expansion in the number of Patients who were scheduled to undergo rou available preparations; indeed until recently, tine cataract surgery were recruited to the estimation of intraocular penetration has study and informed consent was obtained in been reliant upon extrapolation of data from all cases (n=88), Patients with corneal disease animal models (see Watson et ai., 1988, for or inflammatory ocular conditions which bibliography). -

A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India
OPEN ACCESS Freely available online e Journal of Pharmacovigilance ISSN: 2329-6887 Mini Review A Note on Medical Management of Uveitis Apurupa Nedunuri Department of Pharmacology, Osmania University, Hyderabad, India ABSTRACT Uveitis is a moving illness to treat. Corticosteroids have been utilized in the treatment of uveitis for a long time. Immunosuppressives are acquiring force lately in the treatment of uveitis. In this article we present an outline of current treatment of uveitis and the significant discoveries and advances in medications and visual medication conveyance frameworks in the treatment of uveitis. Keywords: Corticosteroids; Immunosuppressives; Medical Management; Uveitis. INTRODUCTION prednisolone acetic acid derivation is multiple times less powerful on a molar premise than betamethasone or dexamethasone, the Uveitis is a potentially sight threatening disease. It may occur due entrance into the cornea of prednisolone acetic acid derivation is to an infection or may be due to an autoimmune etiology. Specific significantly more than betamethasone or dexamethasone. Dosing antimicrobial therapies with or without corticosteroids are used recurrence and the time span the medicine stays in contact with in cases of infectious uveitis. Several drugs are available for the visual surface additionally impacts adequacy. Suspensions have a management of non-infectious uveitis including corticosteroids, immunosuppressive agents, and more recently biologics. The more serious level of calming impact. treatment of uveitis is evolving -

Steroids Topical
Steroids, Topical Therapeutic Class Review (TCR) September 18, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. September -

[email protected]
SAFETY DATA SHEET Revision Date 13-Jul-2016 Version 1 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING Product identifier Product Name Pred Forte Other means of identification Product Code FP61 Synonyms Prednisolone Acetate Recommended use of the chemical and restrictions on use Recommended Use Corticosteroid This safety data sheet is written to provide health, safety and environmental information for people handling this formulated product in the workplace. It is not intended to provide information relevant to medicinal use of the product. In this instance patients should consult prescribing information/package insert/product label or consult their pharmacist or physician. For health and safety information for individual ingredients used during manufacturing, refer to the appropriate safety data sheet for each ingredient. Details of the supplier of the safety data sheet Manufacturer ALLERGAN 400 Interpace Parkway, Morris Corporate Center III Parsippany, NJ 07054, USA +1-800-272-5525 E-mail address [email protected] Emergency telephone number Emergency Telephone Call CHEMTREC Day or Night Within USA or Canada: 1-800-424-9300 Outside USA and Canada: +1-703-741-5970 (collect calls accepted) 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Reproductive toxicity Category 2 Effects on or via lactation Yes Label elements Emergency Overview Danger Hazard statements H362 - May cause harm to breast-fed -
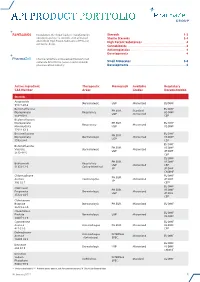
Api Product Portfolio
API PRODUCT PORTFOLIO Farmabios is the global leader in manufacturing Steroids . 1-3 nonsterile and sterile steroids, with a focused Sterile Steroids . 3-4 portfolio of High Potent Substances (HPS) and High Potent Substances . 4 anticancer drugs. Cannabinoids . 4 Antineoplastics . 4 Developments . 4 PharmaZell offers a focused portfolio of small molecule APIs for the generic and originator Small Molecules . 5-6 pharmaceutical industry. Developments . 6 Active Ingredient Therapeutic Monograph Available Regulatory CAS Number Areas Grades Documentation Steroids Amcinonide Dermatologic USP Micronized EU DMF 51022-69-6 Beclomethasone EU DMF PH.EUR. Standard Dipropionate Respiratory US DMF USP Micronized 5534-09-8 CEP Beclomethasone Dipropionate PH.EUR. EU DMF Respiratory Micronized Monohydrate USP US DMF 77011-63-3 Betamethasone EU DMF PH.EUR. Dipropionate Dermatologic Micronized US DMF USP 5593-20-4 CEP EU DMF Betamethasone PH.EUR. US DMF Valerate Dermatologic Micronized USP JP DMF 2152-44-5 CEP EU DMF PH.EUR. US DMF Budesonide Respiratory USP Micronized CEP 51333-22-3 Gastro-Intestinal JP JP DMF CN DMF Chlormadinone EU DMF PH.EUR. Acetate Contraceptive Micronized JP DMF JP 302-22-7 CEP* EU DMF Clobetasol PH.EUR. US DMF Propionate Dermatologic Micronized USP JP DMF 25122-46-7 CEP Clobetasone Butyrate Dermatologic PH.EUR. Micronized EU DMF 25122-57-0 Clocortolone EU DMF Pivalate Dermatologic USP Micronized US DMF 34097-16-0 Cyproterone EU DMF Acetate Anti-Androgen PH.EUR. Micronized US DMF 427-51-0 CEP Delmadinone Anti-Androgen INTERNAL Acetate Micronized EU DMF (Veterinary) SPEC. 13698-49-2 EU DMF Desonide Dermatologic USP Micronized US DMF 638-94-8 CN DMF Desonide Sodium INTERNAL Ophthalmic Standard EU DMF Phosphate SPEC. -

Formulary Updates Effective January 1, 2021
Formulary Updates Effective January 1, 2021 Dear Valued Client, Please see the following lists of formulary updates that will apply to the HometownRx Formulary effective January 1 st , 2021. As the competition among clinically similar products increases, our formulary strategy enables us to prefer safe, proven medication alternatives and lower costs without negatively impacting member choice or access. Please note: Not all drugs listed may be covered under your prescription drug benefit. Certain drugs may have specific restrictions or special copay requirements depending on your plan. The formulary alternatives listed are examples of selected alternatives that are on the formulary. Other alternatives may be available. Members on a medication that will no longer be covered may want to talk to their healthcare providers about other options. Medications that do not have alternatives will be available at 100% member coinsurance . Preferred to Non -Preferred Tier Drug Disease State /Drug Class Preferred Alternatives ALREX Eye inflammation loteprednol (generic for LOTEMAX) APRISO 1 Gastrointestinal agent mesalamine (generic for APRISO) BEPREVE Eye allergies azelastine (generic for OPTIVAR) CIPRODEX 1 Ear inflammation ciprofloxacin-dexamethasone (generic for CIPRODEX) COLCRYS 1 Gout colchicine (generic for COLCRYS) FIRST -LANSOPRAZOLE Gastrointestinal agent Over-the-counter lansoprazole without a prescription FIRST -MOUTHWASH BLM Mouth inflammation lidocaine 2% viscous solution (XYLOCAINE) LOTEMAX 1 Eye inflammation loteprednol etabonate (generic -

Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR)
Ophthalmic Anti-Inflammatories Therapeutic Class Review (TCR) October 20, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004-2020 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Manufacturer Indication(s) Corticosteroids – Ophthalmic Topical dexamethasone (Maxidex®)1 Alcon/Novartis .