Development of CD40-Targeted Bifunctional Scfv-TRAIL Fusion Proteins That Induce TRAILR1- and TRAILR2-Specifc Cell Death and Dendritic Cells Activation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

TNF Decoy Receptors Encoded by Poxviruses
pathogens Review TNF Decoy Receptors Encoded by Poxviruses Francisco Javier Alvarez-de Miranda † , Isabel Alonso-Sánchez † , Antonio Alcamí and Bruno Hernaez * Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Científicas, Campus de Cantoblanco, Universidad Autónoma de Madrid, Nicolás Cabrera 1, 28049 Madrid, Spain; [email protected] (F.J.A.-d.M.); [email protected] (I.A.-S.); [email protected] (A.A.) * Correspondence: [email protected]; Tel.: +34-911-196-4590 † These authors contributed equally. Abstract: Tumour necrosis factor (TNF) is an inflammatory cytokine produced in response to viral infections that promotes the recruitment and activation of leukocytes to sites of infection. This TNF- based host response is essential to limit virus spreading, thus poxviruses have evolutionarily adopted diverse molecular mechanisms to counteract TNF antiviral action. These include the expression of poxvirus-encoded soluble receptors or proteins able to bind and neutralize TNF and other members of the TNF ligand superfamily, acting as decoy receptors. This article reviews in detail the various TNF decoy receptors identified to date in the genomes from different poxvirus species, with a special focus on their impact on poxvirus pathogenesis and their potential use as therapeutic molecules. Keywords: poxvirus; immune evasion; tumour necrosis factor; tumour necrosis factor receptors; lymphotoxin; inflammation; cytokines; secreted decoy receptors; vaccinia virus; ectromelia virus; cowpox virus Citation: Alvarez-de Miranda, F.J.; Alonso-Sánchez, I.; Alcamí, A.; 1. TNF Biology Hernaez, B. TNF Decoy Receptors TNF is a potent pro-inflammatory cytokine with a broad range of biological effects, Encoded by Poxviruses. Pathogens ranging from the activation of inflammatory gene programs to cell differentiation or 2021, 10, 1065. -

New Biological Therapies: Introduction to the Basis of the Risk of Infection
New biological therapies: introduction to the basis of the risk of infection Mario FERNÁNDEZ RUIZ, MD, PhD Unit of Infectious Diseases Hospital Universitario “12 de Octubre”, Madrid ESCMIDInstituto de Investigación eLibraryHospital “12 de Octubre” (i+12) © by author Transparency Declaration Over the last 24 months I have received honoraria for talks on behalf of • Astellas Pharma • Gillead Sciences • Roche • Sanofi • Qiagen Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Paul Ehrlich (1854-1915) • “side-chain” theory (1897) • receptor-ligand concept (1900) • “magic bullet” theory • foundation for specific chemotherapy (1906) • Nobel Prize in Physiology and Medicine (1908) (together with Metchnikoff) Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author 1981: B-1 antibody (tositumomab) anti-CD20 monoclonal antibody 1997: FDA approval of rituximab for the treatment of relapsed or refractory CD20-positive NHL 2001: FDA approval of imatinib for the treatment of chronic myelogenous leukemia Infections and biologicals: a real concern? (two-hour symposium): New biological therapies: introduction to the ESCMIDbasis of the risk of infection eLibrary © by author Functional classification of targeted (biological) agents • Agents targeting soluble immune effector molecules • Agents targeting cell surface receptors -

TRAIL and Cardiovascular Disease—A Risk Factor Or Risk Marker: a Systematic Review
Journal of Clinical Medicine Review TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review Katarzyna Kakareko 1,* , Alicja Rydzewska-Rosołowska 1 , Edyta Zbroch 2 and Tomasz Hryszko 1 1 2nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] (A.R.-R.); [email protected] (T.H.) 2 Department of Internal Medicine and Hypertension, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] * Correspondence: [email protected] Abstract: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a pro-apoptotic protein showing broad biological functions. Data from animal studies indicate that TRAIL may possibly contribute to the pathophysiology of cardiomyopathy, atherosclerosis, ischemic stroke and abdomi- nal aortic aneurysm. It has been also suggested that TRAIL might be useful in cardiovascular risk stratification. This systematic review aimed to evaluate whether TRAIL is a risk factor or risk marker in cardiovascular diseases (CVDs) focusing on major adverse cardiovascular events. Two databases (PubMed and Cochrane Library) were searched until December 2020 without a year limit in accor- dance to the PRISMA guidelines. A total of 63 eligible original studies were identified and included in our systematic review. Studies suggest an important role of TRAIL in disorders such as heart failure, myocardial infarction, atrial fibrillation, ischemic stroke, peripheral artery disease, and pul- monary and gestational hypertension. Most evidence associates reduced TRAIL levels and increased TRAIL-R2 concentration with all-cause mortality in patients with CVDs. It is, however, unclear Citation: Kakareko, K.; whether low TRAIL levels should be considered as a risk factor rather than a risk marker of CVDs. -

B-Cell Targets to Treat Antibody-Mediated Rejection In
Muro et al. Int J Transplant Res Med 2016, 2:023 Volume 2 | Issue 2 International Journal of Transplantation Research and Medicine Commentary: Open Access B-Cell Targets to Treat Antibody-Mediated Rejection in Transplantation Manuel Muro1*, Santiago Llorente2, Jose A Galian1, Francisco Boix1, Jorge Eguia1, Gema Gonzalez-Martinez1, Maria R Moya-Quiles1 and Alfredo Minguela1 1Immunology Service, University Clinic Hospital Virgen de la Arrixaca, Spain 2Nephrology Service, University Clinic Hospital Virgen de la Arrixaca, Spain *Corresponding author: Manuel Muro, PhD, Immunology Service, University Clinic Hospital “Virgen de la Arrixaca”, Biomedical Research Institute of Murcia (IMIB), Murcia, Spain, Tel: 34-968-369599, E-mail: [email protected] Antibody-mediated rejection (AMR) in allograft transplantation APRIL (a proliferation-inducing ligand). These co-activation signals can be defined with a rapid increase in the levels of specific are required for B-cell differentiation into plasma cell and enhancing serological parameters after organ transplantation, presence of donor their posterior survival and are a key determinant of whether specific antibodies (DSAs) against human leukocyte antigen (HLA) developing B-cells will survive or die during the establishment molecules, blood group (ABO) antigens and/or endothelial cell of immuno-tolerance [5,6]. Important used agents commercially antigens (e.g. MICA, ECA, Vimentin, or ETAR) and also particular available are Tocilizumab (anti-IL6R) and Belimumab (BAFF). histological parameters [1,2]. If the AMR persists or progresses, the The receptors of BAFF and APRIL could also be important as treatment to eliminate the humoral component of acute rejection eventual targets, for example BAFF-R, TACI (transmembrane include three sequential steps: (a) steroid pulses, antibody removal activator and calcium modulator and cyclophyllin ligand interactor) (plasma exchange or immuno-adsorption) and high doses of and BCMA (B-cell maturation antigen). -

(12) United States Patent (10) Patent No.: US 9,161,992 B2 Jefferies Et Al
US009 161992B2 (12) United States Patent (10) Patent No.: US 9,161,992 B2 Jefferies et al. (45) Date of Patent: Oct. 20, 2015 (54) P97 FRAGMENTS WITH TRANSFER 4,683.202 A 7, 1987 Mullis ACTIVITY 4,704,362 A 11/1987 Itakura et al. 4,766,075 A 8, 1988 Goeddeletal. (71) Applicant: biosis Technologies, Inc., Richmond 4,800,1594,784.950 A 11/19881/1989 MullisHagen et al. (CA) 4,801,542 A 1/1989 Murray et al. 4.866,042 A 9, 1989 Neuwelt (72) Inventors: Wilfred Jefferies, South Surrey (CA); 4,935,349 A 6/1990 McKnight et al. Mei Mei Tian, Coquitlam (CA): 4.946,778 A 8, 1990 Ladner et al. Timothy Vitalis, Vancouver (CA) 5,091,513 A 2f1992 Huston et al. 5,132,405 A 7, 1992 Huston et al. (73) Assignee: biOasis Technologies, Inc., British 5, 186,941 A 2f1993 Callahan et al. Columbia (CA) 5,672,683 A 9, 1997 Friden et al. 5,677,171 A 10, 1997 Hudziak et al. c - r 5,720,937 A 2f1998 Hudziak et al. (*) Notice: Subject to any disclaimer, the term of this 5,720,954. A 2f1998 Hudziak et al. patent is extended or adjusted under 35 5,725,856 A 3, 1998 Hudziak et al. U.S.C. 154(b) by 0 days. 5,770,195 A 6/1998 Hudziak et al. 5,772,997 A 6/1998 Hudziak et al. (21) Appl. No.: 14/226,506 5,844,093 A 12/1998 Kettleborough et al. 5,962,012 A 10, 1999 Lin et al. -
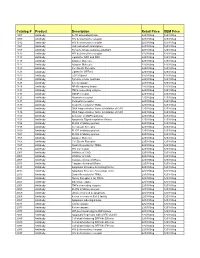
Comprehensive Product List
Catalog # Product Description Retail Price OEM Price 1007 Antibody IL-1R associated kinase 225/100ug 125/100ug 1009 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1012 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1021 Antibody JAK activated transcription 225/100ug 125/100ug 1107 Antibody Tyrosine kinase substrate p62DOK 225/100ug 125/100ug 1112 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1113 Antibody Ligand for DR4 and DR5 225/100ug 125/100ug 1115 Antibody Adapter Molecule 225/100ug 125/100ug 1117 Antibody Adapter Molecule 225/100ug 125/100ug 1120 Antibody Cell Death Receptor 225/100ug 125/100ug 1121 Antibody Ligand for GFRa-2 225/100ug 125/100ug 1123 Antibody CCR3 ligand 225/100ug 125/100ug 1125 Antibody Tyrosine kinase substrate 225/100ug 125/100ug 1128 Antibody A new caspase 225/100ug 125/100ug 1129 Antibody NF-kB inducing kinase 225/100ug 125/100ug 1131 Antibody TNFa converting enzyme 225/100ug 125/100ug 1133 Antibody GDNF receptor 225/100ug 125/100ug 1135 Antibody Neurturin receptor 225/100ug 125/100ug 1137 Antibody Persephin receptor 225/100ug 125/100ug 1139 Antibody Death Receptor for TRAIL 225/100ug 125/100ug 1141 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1148 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1150 Antibody Activator of MAPK pathway 225/100ug 125/100ug 1151 Antibody Apoptosis Signal-regulation Kinase 225/100ug 125/100ug 1156 Antibody FLICE inhibitory protein 225/100ug 125/100ug 1158 Antibody Cell Death Receptor 225/100ug 125/100ug 1159 -

Monoclonal Antibodies in Myeloma
Monoclonal Antibodies in Myeloma Pia Sondergeld, PhD, Niels W. C. J. van de Donk, MD, PhD, Paul G. Richardson, MD, and Torben Plesner, MD Dr Sondergeld is a medical student at the Abstract: The development of monoclonal antibodies (mAbs) for University of Giessen in Giessen, Germany. the treatment of disease goes back to the vision of Paul Ehrlich in Dr van de Donk is a hematologist in the the late 19th century; however, the first successful treatment with department of hematology at the VU a mAb was not until 1982, in a lymphoma patient. In multiple University Medical Center in Amsterdam, The Netherlands. Dr Richardson is the R.J. myeloma, mAbs are a very recent and exciting addition to the Corman Professor of Medicine at Harvard therapeutic armamentarium. The incorporation of mAbs into Medical School, and clinical program current treatment strategies is hoped to enable more effective and leader and director of clinical research targeted treatment, resulting in improved outcomes for patients. at the Jerome Lipper Myeloma Center, A number of targets have been identified, including molecules division of hematologic malignancy, depart- on the surface of the myeloma cell and components of the bone ment of medical oncology, Dana-Farber Cancer Institute in Boston, MA. Dr Plesner marrow microenvironment. Our review focuses on a small number is a professor of hematology at the Univer- of promising mAbs directed against molecules on the surface of sity of Southern Denmark and a consultant myeloma cells, including CS1 (elotuzumab), CD38 (daratumumab, in the department of hematology at Vejle SAR650984, MOR03087), CD56 (lorvotuzumab mertansine), and Hospital in Vejle, Denmark. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Monoclonal Antibody-Based Therapy As a New Treatment Strategy in Multiple Myeloma
Leukemia (2012) 26, 199–213 & 2012 Macmillan Publishers Limited All rights reserved 0887-6924/12 www.nature.com/leu REVIEW Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma NWCJ van de Donk1, S Kamps1, T Mutis2 and HM Lokhorst1 1Department of Hematology, University Medical Center Utrecht, Utrecht, The Netherlands and 2Department of Clinical Chemistry and Hematology, University Medical Center Utrecht, Utrecht, The Netherlands The introduction of autologous stem cell transplantation the myeloma patients achieved a partial response (PR) or stable combined with the introduction of immunomodulatory drugs disease following rituximab therapy. All these patients expressed (IMiDs) and proteasome inhibitors has significantly improved CD20 on their myeloma cells.2 However, as only B15–20% of survival of multiple myeloma patients. However, ultimately the majority of patients will develop refractory disease, indicating all myeloma patients express CD20 on their bone marrow the need for new treatment modalities. In preclinical and clinical plasma cells, new targets for immunotherapy need to be studies, promising results have been obtained with several identified. The search for other targets has led to the develop- monoclonal antibodies (mAbs) targeting the myeloma tumor ment of mAbs targeting growth factor receptors or adhesion cell or the bone marrow microenvironment. The mechanisms molecules on myeloma cells. Other newly developed mAbs underlying the therapeutic efficacy of these mAbs include are directed against cellular or non-cellular components of the direct induction of tumor cell apoptosis via inhibition or activation of target molecules, complement-dependent cyto- bone marrow microenvironment, resulting in the neutraliza- toxicity and antibody-dependent cell-mediated cytotoxicity tion of growth factors, inhibition of angiogenesis, modulation (ADCC). -

Biolegend.Com
Mechanisms of Cell Death TRAIL (TNFSF10) TNF-α Death Receptor 4 (TNFRSF10A/TRAIL-R1) Death Receptor 5 Zombie Dyes (TNFRSF10B/TRAIL-R2) Propidium Iodide (PI) BAT1, TIM-4 TNF RI (TNFRSF1A) 7-Amino-Actinomycin (7-AAD) MER TNF RII (TNFRSF1B) FAS-L GAS6 (TNFSF6/CD178) TRAIL (TNFSF10) Apoptotic Cell Death Domain Zombie Dyes Phosphatidylserine K63 Ubiquitin NH2 Removal ICAM3? ROCK1 NH CD14 2 Eat-Me Signals FAS Death Inducing Cytoskeletal Rearrangement, (TNFRSF6/CD95) Signaling Complex (DISC) TRADD Cytoskeletal Rearrangement, TRADD Decoy Receptor 2 FADD (TNFRSF10D/TRAIL-R4) Actomysin Contraction Engulfment RIP1 TWEAK RIP1 oxLDL (TNFSF12) FADD CIAP1/2 K63 Ubiquitination Blebbing CD36 Death Receptor 3 TWEAK (TNFSF12) PI FADD (TNFRSF25, APO-3) 7-AAD TRAF1 FADD Procaspase 8,10 TRAF 3 Phagocyte FLIP PANX1 Macrophage Monocyte Neutrophil Dendritic Cell Fibroblast Mast Cell Procaspase 8,10 NF-kB TWEAK-R (TNFRSF12A/Fn14) Find-Me Signals Lysophosphocholine C Caspase 8,10 TRAF5 TRAF2 Sphingosine-1-Phosphate G2A? Nucleotides A Decoy TRAIL Receptor R1 (TNFRSF23) Bid Cell Survival ATP, UTP Decoy TRAIL Receptor R2 (TNFRSF22) Sphingosine-1 TRADD Phosphate Receptor Decoy Receptor 1 (TNFRSF10C/TRAIL-R3) Procaspase 3 Proliferation RIP1 G P2y2 t-Bid Bcl-2 T Chemotaxis, Caspase 3 Bcl-2-xL, MCL-1 ? ICAD RIP1 Engulfment Degradation Bax, Bak Oligomerization TRADD Death Receptor 6 Extracellular ATP Bacterial pore-forming toxins TRAIL (TNFSF10) ICAD (TNFRSF21) Monosodium urate crystals Cholesterol crystals Death Receptor DNA Fragmentation Cholera toxin B, Mitochondria