TNF Decoy Receptors Encoded by Poxviruses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JOURNAL of VIROLOGY Volume 18 Contents for May Number 2
JOURNAL OF VIROLOGY Volume 18 Contents for May Number 2 Animal Viruses Isolation and Properties of the Replicase of Encephalomyocarditis Virus. A. TRAUB,* B. DISKIN, H. ROSENBERG, AND E. KALMAR ...... ............... 375 Synthesis and Integration of Viral DNA in Chicken Cells at Different Times After Infection with Various Multiplicities of Avian Oncornavirus. ALLAN T. KHOURY* AND HIDESABURO HANAFUSA ........ .......................... 383 RNA Metabolism of Murine Leukemia Virus. III. Identification and Quantitation of Endogenous Virus-Specific mRNA in the Uninfected BALB/c Cell Line JLS-V9. HUNG FAN AND NIKOLAUS MUELLER-LANTZSCH* ..... ............ 401 Endogenous Ecotropic Mouse Type C Viruses Deficient in Replication and Produc- tion of XC Plaques. ULF W RAPP AND ROBERT C. NoWINSKI* ..... ....... 411 Further Characterization of the Friend Murine Leukemia Virus Reverse Tran- scriptase-RNase H Complex. KARIN MOELLING ...... ................... 418 State of the Viral DNA in Rat Cells Transformed by Polyoma Virus. I. Virus Res- cue and the Presence of Nonintegrated Viral DNA Molecules. ISHWARI PRASAD, DIMITRIS ZOUZIAS, AND CLAUDIO BASIUCO* ...... ................ 436 Inhibition of Infectious Rous Sarcoma Virus Production by a Rifamycin Deriva- tive. CHARLES SZABO,* MINA J. BISSELL, AND MELVIN CALVIN ..... ...... 445 Synthesis ofthe Adenovirus-Coded DNA Binding Protein in Infected Cells. ZVEE GILEAD,* KINJI SUGUWARA, G. SHANMUGAM, AND MAURICE GREEN ....... 454 Intracellular Distribution and Sedimentation Properties of Virus-Specific RNA in Two Clones of BHK Cells Transformed by Polyoma Virus. IAN H. MAX- WELL .............................................................. 461 Inherited Resistance to N- and B-Tropic Murine Leukemia Viruses In Vitro: Titration Patterns in Strains SIM and SIM.R Congenic at the Fv-1 Locus. VERA SCHUH, MARTIN E. BLACKSTEIN, AND ARTHUR A. -

Cytokine Nomenclature
RayBiotech, Inc. The protein array pioneer company Cytokine Nomenclature Cytokine Name Official Full Name Genbank Related Names Symbol 4-1BB TNFRSF Tumor necrosis factor NP_001552 CD137, ILA, 4-1BB ligand receptor 9 receptor superfamily .2. member 9 6Ckine CCL21 6-Cysteine Chemokine NM_002989 Small-inducible cytokine A21, Beta chemokine exodus-2, Secondary lymphoid-tissue chemokine, SLC, SCYA21 ACE ACE Angiotensin-converting NP_000780 CD143, DCP, DCP1 enzyme .1. NP_690043 .1. ACE-2 ACE2 Angiotensin-converting NP_068576 ACE-related carboxypeptidase, enzyme 2 .1 Angiotensin-converting enzyme homolog ACTH ACTH Adrenocorticotropic NP_000930 POMC, Pro-opiomelanocortin, hormone .1. Corticotropin-lipotropin, NPP, NP_001030 Melanotropin gamma, Gamma- 333.1 MSH, Potential peptide, Corticotropin, Melanotropin alpha, Alpha-MSH, Corticotropin-like intermediary peptide, CLIP, Lipotropin beta, Beta-LPH, Lipotropin gamma, Gamma-LPH, Melanotropin beta, Beta-MSH, Beta-endorphin, Met-enkephalin ACTHR ACTHR Adrenocorticotropic NP_000520 Melanocortin receptor 2, MC2-R hormone receptor .1 Activin A INHBA Activin A NM_002192 Activin beta-A chain, Erythroid differentiation protein, EDF, INHBA Activin B INHBB Activin B NM_002193 Inhibin beta B chain, Activin beta-B chain Activin C INHBC Activin C NM005538 Inhibin, beta C Activin RIA ACVR1 Activin receptor type-1 NM_001105 Activin receptor type I, ACTR-I, Serine/threonine-protein kinase receptor R1, SKR1, Activin receptor-like kinase 2, ALK-2, TGF-B superfamily receptor type I, TSR-I, ACVRLK2 Activin RIB ACVR1B -

Dimerization of Ltβr by Ltα1β2 Is Necessary and Sufficient for Signal
Dimerization of LTβRbyLTα1β2 is necessary and sufficient for signal transduction Jawahar Sudhamsua,1, JianPing Yina,1, Eugene Y. Chiangb, Melissa A. Starovasnika, Jane L. Groganb,2, and Sarah G. Hymowitza,2 Departments of aStructural Biology and bImmunology, Genentech, Inc., South San Francisco, CA 94080 Edited by K. Christopher Garcia, Stanford University, Stanford, CA, and approved October 24, 2013 (received for review June 6, 2013) Homotrimeric TNF superfamily ligands signal by inducing trimers survival in a xenogeneic human T-cell–dependent mouse model of of their cognate receptors. As a biologically active heterotrimer, graft-versus-host disease (GVHD) (11). Lymphotoxin(LT)α1β2 is unique in the TNF superfamily. How the TNFRSF members are typically activated by TNFSF-induced three unique potential receptor-binding interfaces in LTα1β2 trig- trimerization or higher order oligomerization, resulting in initiation ger signaling via LTβ Receptor (LTβR) resulting in lymphoid organ- of intracellular signaling processes including the canonical and ogenesis and propagation of inflammatory signals is poorly noncanonical NF-κB pathways (2, 3). Ligand–receptor interactions α β understood. Here we show that LT 1 2 possesses two binding induce higher order assemblies formed between adaptor motifs in sites for LTβR with distinct affinities and that dimerization of LTβR the cytoplasmic regions of the receptors such as death domains or α β fi by LT 1 2 is necessary and suf cient for signal transduction. The TRAF-binding motifs and downstream signaling components such α β β crystal structure of a complex formed by LT 1 2,LT R, and the fab as Fas-associated protein with death domain (FADD), TNFR1- fragment of an antibody that blocks LTβR activation reveals the associated protein with death domain (TRADD), and TNFR-as- lower affinity receptor-binding site. -

The Unexpected Role of Lymphotoxin Β Receptor Signaling
Oncogene (2010) 29, 5006–5018 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 www.nature.com/onc REVIEW The unexpected role of lymphotoxin b receptor signaling in carcinogenesis: from lymphoid tissue formation to liver and prostate cancer development MJ Wolf1, GM Seleznik1, N Zeller1,3 and M Heikenwalder1,2 1Department of Pathology, Institute of Neuropathology, University Hospital Zurich, Zurich, Switzerland and 2Institute of Virology, Technische Universita¨tMu¨nchen/Helmholtz Zentrum Mu¨nchen, Munich, Germany The cytokines lymphotoxin (LT) a, b and their receptor genesis. Consequently, the inflammatory microenviron- (LTbR) belong to the tumor necrosis factor (TNF) super- ment was added as the seventh hallmark of cancer family, whose founder—TNFa—was initially discovered (Hanahan and Weinberg, 2000; Colotta et al., 2009). due to its tumor necrotizing activity. LTbR signaling This was ultimately the result of more than 100 years of serves pleiotropic functions including the control of research—indeed—the first observation that tumors lymphoid organ development, support of efficient immune often arise at sites of inflammation was initially reported responses against pathogens due to maintenance of intact in the nineteenth century by Virchow (Balkwill and lymphoid structures, induction of tertiary lymphoid organs, Mantovani, 2001). Today, understanding the underlying liver regeneration or control of lipid homeostasis. Signal- mechanisms of why immune cells can be pro- or anti- ing through LTbR comprises the noncanonical/canonical carcinogenic in different types of tumors and which nuclear factor-jB (NF-jB) pathways thus inducing cellular and molecular inflammatory mediators (for chemokine, cytokine or adhesion molecule expression, cell example, macrophages, lymphocytes, chemokines or proliferation and cell survival. -

Escherichia Coli Saccharomyces Cerevisiae Bacillus Subtilis はB
研究開発等に係る遺伝子組換え生物等の第二種使用等に当たって執るべき拡散防止措 置等を定める省令の規定に基づき認定宿主ベクター系等を定める件 (平成十六年一月二十九日文部科学省告示第七号) 最終改正:令和三年二月十五日文部科学省告示第十三号 (認定宿主ベクター系) 第一条 研究開発等に係る遺伝子組換え生物等の第二種使用等に当たって執るべき拡散防止 措置等を定める省令(以下「省令」という。)第二条第十三号の文部科学大臣が定める認 定宿主ベクター系は、別表第一に掲げるとおりとする。 (実験分類の区分ごとの微生物等) 第二条 省令第三条の表第一号から第四号までの文部科学大臣が定める微生物等は、別表第 二の上欄に掲げる区分について、それぞれ同表の下欄に掲げるとおりとする。 (特定認定宿主ベクター系) 第三条 省令第五条第一号ロの文部科学大臣が定める特定認定宿主ベクター系は、別表第一 の2の項に掲げる認定宿主ベクター系とする。 (自立的な増殖力及び感染力を保持したウイルス及びウイロイド) 第四条 省令別表第一第一号ヘの文部科学大臣が定めるウイルス及びウイロイドは、別表第 三に掲げるとおりとする。 別表第1(第1条関係) 区 分 名 称 宿主及びベクターの組合せ 1 B1 (1) EK1 Escherichia coli K12株、B株、C株及びW株又は これら各株の誘導体を宿主とし、プラスミド又は バクテリオファージの核酸であって、接合等によ り宿主以外の細菌に伝達されないものをベクター とするもの(次項(1)のEK2に該当するものを除 く。) (2) SC1 Saccharomyces cerevisiae又はこれと交雑可能な 分類学上の種に属する酵母を宿主とし、これらの 宿主のプラスミド、ミニクロモソーム又はこれら の誘導体をベクターとするもの(次項(2)のSC2 に該当するものを除く。) (3) BS1 Bacillus subtilis Marburg168株、この誘導体又 はB. licheniformis全株のうち、アミノ酸若しく は核酸塩基に対する複数の栄養要求性突然変異を 有する株又は胞子を形成しない株を宿主とし、こ れらの宿主のプラスミド(接合による伝達性のな いものに限る。)又はバクテリオファージの核酸 をベクターとするもの(次項(3)のBS2に該当す るものを除く。) (4) Thermus属細菌 Thermus属細菌(T. thermophilus、T. aquaticus、 T. flavus、T. caldophilus及びT. ruberに限る。) を宿主とし、これらの宿主のプラスミド又はこの 誘導体をベクターとするもの (5) Rhizobium属細菌 Rhizobium属細菌(R. radiobacter(別名Agroba- cterium tumefaciens)及びR. rhizogenes(別名 Agrobacterium rhizogenes)に限る。)を宿主と し、これらの宿主のプラスミド又はRK2系のプラ スミドをベクターとするもの (6) Pseudomonas putida Pseudomonas putida KT2440株又はこの誘導体を 宿主とし、これら宿主への依存性が高く、宿主以 外の細胞に伝達されないものをベクターとするも の (7) Streptomyces属細菌 Streptomyces属細菌(S. avermitilis、S. coel- icolor [S. violaceoruberとして分類されるS. coelicolor A3(2)株を含む]、S. lividans、S. p- arvulus、S. griseus及びS. -

TRAIL and Cardiovascular Disease—A Risk Factor Or Risk Marker: a Systematic Review
Journal of Clinical Medicine Review TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review Katarzyna Kakareko 1,* , Alicja Rydzewska-Rosołowska 1 , Edyta Zbroch 2 and Tomasz Hryszko 1 1 2nd Department of Nephrology and Hypertension with Dialysis Unit, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] (A.R.-R.); [email protected] (T.H.) 2 Department of Internal Medicine and Hypertension, Medical University of Białystok, 15-276 Białystok, Poland; [email protected] * Correspondence: [email protected] Abstract: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a pro-apoptotic protein showing broad biological functions. Data from animal studies indicate that TRAIL may possibly contribute to the pathophysiology of cardiomyopathy, atherosclerosis, ischemic stroke and abdomi- nal aortic aneurysm. It has been also suggested that TRAIL might be useful in cardiovascular risk stratification. This systematic review aimed to evaluate whether TRAIL is a risk factor or risk marker in cardiovascular diseases (CVDs) focusing on major adverse cardiovascular events. Two databases (PubMed and Cochrane Library) were searched until December 2020 without a year limit in accor- dance to the PRISMA guidelines. A total of 63 eligible original studies were identified and included in our systematic review. Studies suggest an important role of TRAIL in disorders such as heart failure, myocardial infarction, atrial fibrillation, ischemic stroke, peripheral artery disease, and pul- monary and gestational hypertension. Most evidence associates reduced TRAIL levels and increased TRAIL-R2 concentration with all-cause mortality in patients with CVDs. It is, however, unclear Citation: Kakareko, K.; whether low TRAIL levels should be considered as a risk factor rather than a risk marker of CVDs. -

The Function of CD27 Costimulation in the Activation and Fate Decisions of CD8+ T Cells
The function of CD27 costimulation in the activation and fate decisions of CD8+ T cells Han Dong Zhengzhou, Henan Province, China B.Sc, Zhejing University, 2009 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Experimental Pathology University of Virginia May 2015 ! i! Abstract CD8+ cytotoxic T lymphocytes are critical components of adaptive immunity against a variety of intracellular pathogens, and can play a key role in the control of tumors. Effective vaccination strategies against viral infections and tumors will likely require the development of potent CD8+ T cell responses, which are constituted by the expansion of robust primary CD8+ T cell populations and the establishment of long-lasting memory. Fully functional CD8+ T cell responses are highly dependent upon CD4+ helper T cells and Signal 3 inflammatory cytokine pathways. CD4+ T cells have been demonstrated to play a critical role in inducing the expression of CD70, the ligand for CD27, on dendritic cells. However, it is not clear to what extent the ‘help’ provided by CD4+ T cells is manifest via CD70, or how CD70-mediated stimulation of CD8+ T cells is integrated with signals that emanate from Signal 3 pathways, such as type-1 interferon (IFN-1) and IL- 12. In this work, by enforcing or abrogating CD27 function by genetic or protein intervention in murine models, we sought to identify the function of CD27 costimulation in the activation and fate decisions of CD8+ T cells, to determine the extent it resembles CD4+ T cell help, and how inflammation impacts the relative importance of CD70-CD27 interactions in CD8+ T cell primary responses and CD8+ T cell memory. -
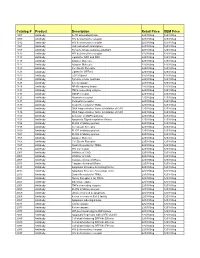
Comprehensive Product List
Catalog # Product Description Retail Price OEM Price 1007 Antibody IL-1R associated kinase 225/100ug 125/100ug 1009 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1012 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1021 Antibody JAK activated transcription 225/100ug 125/100ug 1107 Antibody Tyrosine kinase substrate p62DOK 225/100ug 125/100ug 1112 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1113 Antibody Ligand for DR4 and DR5 225/100ug 125/100ug 1115 Antibody Adapter Molecule 225/100ug 125/100ug 1117 Antibody Adapter Molecule 225/100ug 125/100ug 1120 Antibody Cell Death Receptor 225/100ug 125/100ug 1121 Antibody Ligand for GFRa-2 225/100ug 125/100ug 1123 Antibody CCR3 ligand 225/100ug 125/100ug 1125 Antibody Tyrosine kinase substrate 225/100ug 125/100ug 1128 Antibody A new caspase 225/100ug 125/100ug 1129 Antibody NF-kB inducing kinase 225/100ug 125/100ug 1131 Antibody TNFa converting enzyme 225/100ug 125/100ug 1133 Antibody GDNF receptor 225/100ug 125/100ug 1135 Antibody Neurturin receptor 225/100ug 125/100ug 1137 Antibody Persephin receptor 225/100ug 125/100ug 1139 Antibody Death Receptor for TRAIL 225/100ug 125/100ug 1141 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1148 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1150 Antibody Activator of MAPK pathway 225/100ug 125/100ug 1151 Antibody Apoptosis Signal-regulation Kinase 225/100ug 125/100ug 1156 Antibody FLICE inhibitory protein 225/100ug 125/100ug 1158 Antibody Cell Death Receptor 225/100ug 125/100ug 1159 -

CD95 Ligand - Death Factor and Costimulatory Molecule?
Cell Death and Differentiation (2003) 10, 1215–1225 & 2003 Nature Publishing Group All rights reserved 1350-9047/03 $25.00 www.nature.com/cdd Review CD95 ligand - death factor and costimulatory molecule? O Janssen*,1, J Qian1, A Linkermann1 and D Kabelitz1 Tissue and Cellular Expression of CD95L 1 Institute for Immunology, Medical Center Schleswig-Holstein, Campus Kiel, Michaelisstrasse 5, D-24105 Kiel, Germany The CD95 ligand (CD95L, Apo-1L, FasL, CD178) is a 281- * Corresponding author: O Janssen. Tel: þ 49-431-5973377; Fax: þ 49-431- amino-acid-containing type II transmembrane protein of the 5973335; E-mail: [email protected] TNF family of death factors (Figure 1).1 Its death-inducing function is best documented in the context of activation- Received 24.4.03; revised 12.6.03; accepted 20.6.03; published online 1 August 2003 induced cell death (AICD) in T cells.2 CD95L is expressed as a Edited by T Ferguson death factor in cytotoxic T lymphocytes (CTL) to kill virally infected or transformed target cells and in natural killer (NK) cells, where it is upregulated by CD16 engagement and 3 Abstract cytokines including IL-2 and IL-12. Similarly, high levels of intracellular CD95L have been detected in monocytic cells The CD95 ligand is involved as a death factor in the with an inducible release upon activation.4 Under physiologi- regulation of activation-induced cell death, establishment cal conditions, CD95L is implicated in the control of erythroid of immune privilege and tumor cell survival. In addition, differentiation,5 angiogenesis in the eye6 and skin home- 7 CD95L may serve as a costimulatory molecule for T-cell ostasis. -

Due to Interleukin-6 Type Cytokine Redundancy Only Glycoprotein 130 Receptor Blockade Efficiently Inhibits Myeloma Growth
Plasma Cell Disorders SUPPLEMENTARY APPENDIX Due to interleukin-6 type cytokine redundancy only glycoprotein 130 receptor blockade efficiently inhibits myeloma growth Renate Burger, 1 Andreas Günther, 1 Katja Klausz, 1 Matthias Staudinger, 1 Matthias Peipp, 1 Eva Maria Murga Penas, 2 Stefan Rose-John, 3 John Wijdenes 4 and Martin Gramatzki 1 1Division of Stem Cell Transplantation and Immunotherapy, Department of Internal Medicine II, Christian-Albrechts-University Kiel and University Medical Center Schleswig-Holstein, Kiel, Germany; 2Institute of Human Genetics, Christian-Albrechts-University Kiel and Uni - versity Medical Center Schleswig-Holstein, Kiel, Germany; 3Department of Biochemistry, Christian-Albrechts-University of Kiel, Medical Faculty, Germany and 4Gen-Probe/Diaclone SAS, Besançon, France ©2017 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol. 2016.145060 Received: February 25, 2016. Accepted: September 14, 2016. Pre-published: September 22, 2016. Correspondence: [email protected] SUPPLEMENTARY METHODS Cell lines and culture Cell lines INA-6, INA-6.Tu1 and B9 were cultivated in RPMI-1640 with GlutaMax™-I, 25 mM HEPES (Gibco®/Life Technologies GmbH, Darmstadt, Germany), 10% (v/v) heat-inactivated fetal bovine serum (FBS) (HyClone; Perbio Science, Erembodegen, Belgium), and antibiotics (R10+ medium) supplemented with 2.5 ng/ml recombinant huIL-6 (Gibco®/Life Technologies GmbH, Darmstadt, Germany). The cell lines are routinely confirmed to be negative for mycoplasma contamination (Venor™GeM Mycoplasma Detection Kit, Sigma-Aldrich, St. Louis, MO). Cytokines and other reagents Recombinant huIL-6 was purchased from Gibco®/Life Technologies (Darmstadt, Germany), huLIF was from Reliatech (Wolfenbüttel, Germany). Recombinant muIL-6 was obtained from Peprotech (Rocky Hill, NJ), and soluble muIL-6R was from R&D Systems (Minneapolis, MN). -

Decoy Strategies: the Structure of TL1A:Dcr3 Complex
Structure Article Decoy Strategies: The Structure of TL1A:DcR3 Complex Chenyang Zhan,1 Yury Patskovsky,1 Qingrong Yan,2 Zhenhong Li,1 Udupi Ramagopal,1 Huiyong Cheng,1 Michael Brenowitz,1 Xiao Hui,3 Stanley G. Nathenson,2,4 and Steven C. Almo1,5,* 1Department of Biochemistry 2Department of Cell Biology 3Department of Developmental and Molecular Biology 4Department of Microbiology and Immunology 5Department of Physiology and Biophysics Albert Einstein College of Medicine, Bronx, NY 10461, USA *Correspondence: [email protected] DOI 10.1016/j.str.2010.12.004 SUMMARY domains (CRDs), which bind the interprotomer grooves formed between adjacent ligand monomers. This mode of interaction Decoy Receptor 3 (DcR3), a secreted member of the results in ligand:receptor assemblies with 3:3 stoichiometries Tumor Necrosis Factor (TNF) receptor superfamily, that span two interacting cells. The ligand-mediated clustering neutralizes three different TNF ligands: FasL, LIGHT, of TNFRs directs the recruitment of signaling adaptor proteins, and TL1A. Each of these ligands engages unique such as TNF receptor associated factors (TRAFs) or death signaling receptors which direct distinct and critical domain adaptor proteins, and initiates diverse downstream immune responses. We report the crystal structures signaling pathways. Some TNFRs are also produced as soluble forms due to alter- of the unliganded DcR3 ectodomain and its complex nate mRNA splicing or selective proteolytic cleavage (Cheng with TL1A, as well as complementary mutagenesis et al., 1994; Locksley et al., 2001; Van Zee et al., 1992). The met- and biochemical studies. These analyses demon- alloprotease-mediated cleavage of two TNF-a receptors (TNFR1 strate that DcR3 interacts with invariant backbone and TNFR2) has been reported to attenuate proinflammatory and side-chain atoms in the membrane-proximal TNF-a-associated signaling, possibly by reducing the amount half of TL1A which supports recognition of its three of cell surface receptors and by the sequestration/neutralization distinct TNF ligands. -

ICTV Code Assigned: 2011.001Ag Officers)
This form should be used for all taxonomic proposals. Please complete all those modules that are applicable (and then delete the unwanted sections). For guidance, see the notes written in blue and the separate document “Help with completing a taxonomic proposal” Please try to keep related proposals within a single document; you can copy the modules to create more than one genus within a new family, for example. MODULE 1: TITLE, AUTHORS, etc (to be completed by ICTV Code assigned: 2011.001aG officers) Short title: Change existing virus species names to non-Latinized binomials (e.g. 6 new species in the genus Zetavirus) Modules attached 1 2 3 4 5 (modules 1 and 9 are required) 6 7 8 9 Author(s) with e-mail address(es) of the proposer: Van Regenmortel Marc, [email protected] Burke Donald, [email protected] Calisher Charles, [email protected] Dietzgen Ralf, [email protected] Fauquet Claude, [email protected] Ghabrial Said, [email protected] Jahrling Peter, [email protected] Johnson Karl, [email protected] Holbrook Michael, [email protected] Horzinek Marian, [email protected] Keil Guenther, [email protected] Kuhn Jens, [email protected] Mahy Brian, [email protected] Martelli Giovanni, [email protected] Pringle Craig, [email protected] Rybicki Ed, [email protected] Skern Tim, [email protected] Tesh Robert, [email protected] Wahl-Jensen Victoria, [email protected] Walker Peter, [email protected] Weaver Scott, [email protected] List the ICTV study group(s) that have seen this proposal: A list of study groups and contacts is provided at http://www.ictvonline.org/subcommittees.asp .