Harmoneerituks Tunnistatud Standardid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nummernverzeichnis Der Behandelten Normen
Nummernverzeichnis der behandelten Normen DIN Seite DIN Seite DIN Seite DIN 6-1 83 DIN 244 250 DIN 669 497 DIN 6-2 83 DIN 254* 94 DIN 670 497 DIN 13 252 DIN 267-2 339,340 DIN 671 497 DIN 13-1* 253 DIN 267-6 340 DIN 705 188 DIN 13-2* 253 DIN 267-10 340 DIN 720 189 DIN 13-3* 253 DIN 267-13 340 DIN 747 169 DIN 13-4* 253 DIN 267-24 340 DIN 748 170 DIN 13-5* 253 DIN 316 370 DIN 748-3 853 DIN 13-6* 254 DIN 319 237 DIN 780-1 209 DIN 13-7* 255 DIN 322 205 DIN 780-2 209 DIN 13-8* 255 DIN 323-1 20 DIN 781 272 DIN 13-9* 255 DIN 323-2 20 DIN 803 273 DIN 13-10* 255 DIN 332 148 DIN 804 274 DIN 13-11* 255 DIN 336 266 DIN 820-1 14,17 DIN 34* 82 DIN 405-1* 266 DIN 820-2 18,19 DIN 39 236 DIN 406-10 89 DIN 820-4 17 DIN 66 357 DIN 406-11 89 DIN 820-12 1025 DIN 74-1* 358 DIN 406-12 89 DIN 824 82 DIN 76-1 143 DIN 432 394 DIN 835 367 DIN 78 145 DIN 442 241 DIN 876 208 DIN 79 164 DIN 443 241 DIN 908 372 DIN 82 148 DIN 444 371 DIN 909 372 DIN 93 393 DIN 461 136 DIN 910 372 DIN 95 377 DIN 463 394 DIN 913 368 DIN 96 377 DIN 470 241 DIN 914 368 DIN 97 377 DIN 471 395 DIN 915 368 DIN 98* 236 DIN 472 395 DIN 916 368 DIN 99* 238 DIN 475 164 DIN 935 387 DIN 102 336 DIN 475-1 164 DIN 935-1* 387 DIN 103-1 263 DIN 475-2 164 DIN 938-2* 367 DIN 103-2 263 DIN 476-1 78 DIN 950* 234 DIN 103-3 263 DIN 476-2 78 DIN 974-1 359 DIN 103-4 263 DIN 502 206 DIN 974-2 359 DIN 109-1 173 DIN 504 206 DIN 979 387 DIN 109-2 173 DIN 505 206 DIN 988 207 DIN 111 173 DIN 509 149 DIN 1013-1 489 DIN 115-1 176 DIN 513-1 265 DIN 1013-2 489 DIN 115-2 176 DIN 513-2 265 DIN 1014-1 489 DIN 116 174 DIN -

ISO Focus, June 2008.Pdf
ISO Focus The Magazine of the International Organization for Standardization Volume 5, No. 6, June 2008, ISSN 1729-8709 anica ch l e i M n n o v a t i o n • Bosch : International Standards open up markets • Training trainers in developing countries Contents 1 Comment H. Glenn Ziegenfuss, Executive Director, Standards Engineering Society and Standards Officer, IIW 2 World Scene Highlights of events from around the world 3 ISO Scene Highlights of news and developments from ISO members 4 Guest View Siegfried Dais, Deputy Chairman of the Board of Management ISO Focus is published 11 times a year (single issue : July-August). of Robert Bosch GmbH It is available in English. 8 Main Focus Annual subscription 158 Swiss Francs Individual copies 16 Swiss Francs Publisher nic ISO Central Secretariat ha al (International Organization for c Standardization) e 1, ch. de la Voie-Creuse i CH-1211 Genève 20 M n Switzerland n Telephone + 41 22 749 01 11 o Fax + 41 22 733 34 30 v E-mail [email protected] a o n Web www.iso.org t i Manager : Roger Frost Acting Editor : Maria Lazarte Assistant Editor : Janet Maillard • Strike the first arc and attempt the perfect weld – Artwork : Pascal Krieger and How welding is changing the world Pierre Granier • Globally qualified welders ISO Update : Dominique Chevaux • Safety valves – Vital protection against excessive pressure Subscription enquiries : Sonia Rosas Friot • Performance-based innovation for boilers and pressure vessels ISO Central Secretariat • Reliable testing of metallic materials Telephone + 41 22 749 03 36 • Cutting costs for cutting tools Fax + 41 22 749 09 47 E-mail [email protected] • Springs – From the Stone Age to the Nano Age • Advanced gas turbine technology for power generation – © ISO, 2008. -

HEGESZTÉS Érvényes Szabványok Jegyzéke
HEGESZTÉS érvényes szabványok jegyzéke I. rész: Fogalmak, tervezési és kivitelezési előírások. 1./ Terminológia: MSZ CEN ISO/TS 17845:2005 Hegesztés és rokon eljárásai. Az eltérések jelölési rendszere. 25.160.10 MSZ EN ISO 4063:2000 Hegesztés és rokon eljárások. A hegesztési eljárások megnevezése és azonosító jelölésük. 25.160.10; 01.040.25 - Az MSZ EN 24063:1999 helyett. - Jegyzékes jóváhagyó közleménnyel bevezetett angol nyelvű szabvány Magyar nyelvű változat. 2003/1. SzK. MSZ EN ISO 6520-1:2008 Hegesztési eljárások. A fémek geometriai eltéréseinek osztályba sorolása. 1. rész: Ömlesztőhegesztés. 25.160.40 – Az MSZ EN ISO 6520-1:1999 helyett - Címoldalas jóváhagyó közleménnyel bevezetett angol nyelvű szabvány Magyar nyelvű változat: 2009/07. SzK. MSZ EN ISO 6520-2:2002 Hegesztés és rokon eljárások. Fémek geometriai eltéréseinek besorolása. 2. rész: Sajtolóhegesztés. – Az MSZ EN 26520:1994 helyett.- 25.160.40 Jegyzékes jóváhagyó közleménnyel bevezetett angol nyelvű szabvány MSZ EN ISO 6947:2000 Varratok. Hegesztési helyzetek. A dőlési és az elfordulási szög meghatározása. 01.040.25 – Az MSZ ISO 6947:1990 helyett. - Jegyzékes jóváhagyó közleménnyel bevezetett angol nyelvű szabvány Magyar nyelvű változat megjelent 2001 októberben. Helyesb. 2007/7. SzK. MSZ EN ISO 7287:2002 Lángvágó berendezések grafikus jelképei. – Az MSZ EN ISO 7287:1999 helyett. 01.080.20 Jegyzékes jóváhagyó közleménnyel bevezetett angol nyelvű szabvány MSZ EN ISO 9013:2003 Termikus vágás. A termikusan vágott felületek osztályba sorolása. Geometriai követelmények és minőségi tűrések. – Az MSZ EN ISO 9013:1999 helyett. - 2 Jegyzékes jóváhagyó közleménnyel bevezetett angol nyelvű szabvány MSZ EN ISO 13916:2000 Hegesztés. Irányelvek az előmelegítési, a közbenső és a hőn tartási hőmérséklet mérésére. -

Progress File (Standards Publications)
IRISH STANDARDS PUBLISHED BASED ON CEN/CENELEC STANDARDS 1. I.S. EN 2336:1988/AC1:1988 Date published 1 JANUARY 2006 Aerospace Series - Bearings, Spherical Plain in Steel with Assembly Slots - Dimensions and Loads 2. I.S. EN 28378-1:1989/AC1:1989 Date published 1 JANUARY 2006 Information Processing - Data Interchange on 130 mm (5.25") Flexible Disk Cartridges Using Modified Frequency Modulation Recording at 7958 ftprad, 3.8 tpmm (96 tpi), on Both Sides - Part 1: Dimensional, Physical and Magnetic Characteristics. (ISO 8378-1, 1st Edition, 3. I.S. HD 1215-2:1988/AC1:1989 Date published 1 JANUARY 2006 Thermostatic Radiator Valves - Part 2 : Dimensions and Details on Connection 4. I.S. EN 50203:1996/A1:1999 Date published 2 FEBRUARY 2006 Automatic channel installation (ACI) 5. I.S. EN 54-2:1999 Date published 1 JANUARY 2006 Fire detection and fire alarm systems - Part 2: Control and indicating equipment 6. I.S. EN 12737:2004 Date published 28 JULY 2006 Precast concrete products - Floor slats for livestock 7. I.S. EN 61009-1:1994/A13:2004 Date published 2 FEBRUARY 2006 Electrical accessories - Residual current operated circuit-breakers with integral overcurrent protection for household and similar uses (RCBO's) -- Part 1: General rules 8. I.S. EN 50091-1-1:2004 Date published 2 FEBRUARY 2006 Uninterruptible power systems (UPS) -- Part 1-1: General and safety requirements for UPS used in operator access areas 9. I.S. EN 60432-1:2004 Date published 2 FEBRUARY 2006 Incandescent lamps - Safety specifications -- Part 1: Tungsten filament lamps for domestic and similar general lighting purposes (IEC 60432-1:1999 (MOD)) 10. -

Bulgaria (IGB) Project
“ICGB” AD Consortium FEED & EIA for Natural Gas Interconnector Greece – Bulgaria (IGB) Project List of Applicable Norms and Legislations Penspen Limited 3 Water Lane Richmond upon Thames Surrey TW9 1TJ United Kingdom C&M Engineering SA 99 Pratinou Str. 116 34 Athens Greece 10760-LST-EN-00-001 Rev 0 FEED AND EIA FOR NATURAL GAS Consortium “ICGB” AD INTERCONNECTOR GREECE-BULGARIA (IGB) PROJECT LIST OF APPLICABLE NORMS AND LEGISLATIONS 1. Introduction 1.1 Project Description ICGB AD is a company set up and existing under Bulgarian law and hereafter known as the CONTRACTING ENTITY. The CONTRACTING ENTITY awarded the consortium of Penspen Ltd and C&M Engineering SA, known hereafter as the CONTRACTOR with the Front End Engineering Design (FEED) and Environmental Impact Assessment (EIA) for a proposed 32” diameter high pressure gas pipeline, called the “Gas Interconnector, Greece – Bulgaria” (IGB). The IGB buried pipeline will transport natural gas over the border between Greece and Bulgaria, connecting the existing Komotini Facility in Greece with an existing gas pipeline near the Bulgarian town of Stara Zagora. The proposed pipeline will measure a total distance of approximately 181.3km, (30.5km in Greece and 150.8km in Bulgaria). The design of this bi-directional pipeline system shall be in accordance with the internationally recognised codes of practice: EN1594 and ASME B31.8, and also in conjunction with Bulgarian Ordinances, for the safe transportation of 3bcm/yr of gas initially, with the provision for the future expansion up to a maximum technical capacity of 5bcm/yr. The project also includes the construction of the associated Above Ground Installations (AGIs). -
Applicable Codes and Standards
September 2013 1 The following list of Codes and Standards is intended to be indicative only of the extent to which guidance should be taken from internationally available Codes. This list will not be taken to constitute a complete and exhaustive reference. CODES & STANDARDS FOR PIPELINE/PIPING / MECHANICAL Reference Description PED 97/23/EC Pressure Equipment Directive API RP 1102 Steel Pipelines: Crossings of Railroads and Highways ASME B 31.8 Gas Transmission and Distribution Piping Systems EN 287-1 Qualification on test of welders. Fusion welding. Steels EN 288-9 Approval of welding procedures for metallic materials. Non-destructive testing – Qualification and certification of NDT personnel – EN 473 General principles EN 571-1 Non – Destructive Testing – Penetrant testing – General Principles EN 970 Non-destructive examination of fusion welds-visual examination Flanges and their joints. Circular flanges for pipes, valves, fittings and EN 1092-1 accessories, PN designated. Steel flanges. Non-destructive examination of welds. Penetrant testing of welds – EN 1289 Acceptance levels Non-destructive examination of welds. Magnetic particle examination of EN 1290 welds Non-destructive examination of welds. Magnetic particle examination of EN 1291 welds - Acceptance levels Non – Destructive Examination of welds – Radiographic examination of weld EN 1435 joints Flanges and their joints – Dimensions of gaskets for PN designated flanges – EN 1514-1 & -2 Part 1: Non-metallic flat gaskets with or without inserts, Part 2: Spiral wound gaskets for -
Iso 14555:2017
Irish Standard I.S. EN ISO 14555:2017 Welding - Arc stud welding of metallic materials (ISO 14555:2017) © CEN 2017 No copying without NSAI permission except as permitted by copyright law. This is a free 17 page sample. Access the full version online. I.S. EN ISO 14555:2017 Incorporating amendments/corrigenda/National Annexes issued since publication: The National Standards Authority of Ireland (NSAI) produces the following categories of formal documents: I.S. xxx: Irish Standard — national specification based on the consensus of an expert panel and subject to public consultation. S.R. xxx: Standard Recommendation — recommendation based on the consensus of an expert panel and subject to public consultation. SWiFT xxx: A rapidly developed recommendatory document based on the consensus of the participants of an NSAI workshop. This document replaces/revises/consolidates the NSAI adoption of the document(s) indicated on the CEN/CENELEC cover/Foreword and the following National document(s): NOTE: The date of any NSAI previous adoption may not match the date of its original CEN/CENELEC document. This document is based on: Published: EN ISO 14555:2017 2017-06-14 This document was published ICS number: under the authority of the NSAI and comes into effect on: 25.160.10 2017-07-02 NOTE: If blank see CEN/CENELEC cover page NSAI T +353 1 807 3800 Sales: This is a free 17 page sample. Access the full version online. 1 Swift Square, F +353 1 807 3838 T +353 1 857 6730 Northwood, Santry E [email protected] F +353 1 857 6729 Dublin 9 W NSAI.ie W standards.ie Údarás um Chaighdeáin Náisiúnta na hÉireann National Foreword I.S. -

HZN Oglasnik Za Normativne Dokumente 7
Hrvatski zavod za norme Oglasnik za normativne dokumente 7/2017 srpanj, 2017. Oglasnik za normativne dokumente Hrvatskog zavoda za norme sadrži popise hrvatskih norma, nacrta hrvatskih norma, prijedloga za prihvaćanje stranih norma u izvorniku, povučene hrvatske norme, povučene nacrte hrvatskih norma te ispravke, rezultate europske i međunarodne normizacije razvrstane po predmetnom ustroju i obavijesti HZN-a. Tko u popisima normativnih dokumenata koji su objavljeni u ovom Oglasniku otkrije koju grešku, koja može voditi do krive primjene, moli se da o tome neodložno obavijesti Hrvatski zavod za norme, kako bi se mogli otkloniti uočeni propusti. Izdavač: Sadržaj: 1 Rezultati hrvatske normizacije .................................................................................................. A3 1.1 Hrvatske norme ....................................................................................................................................... A3 1. Nacrti hrvatskih norma ............................................................................................................................ 1.3 Prijedlozi za prihvaćanje stranih norma u izvorniku ................................................................................ A10 1.4 Povučene hrvatske norme ...................................................................................................................... A0 1.5 Povučeni nacrti hrvatskih norma ............................................................................................................. 1.6 Ispravci -
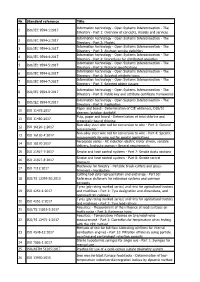
Nr. Standard Reference Title 1 ISO/IEC 9594-1:2017 Information Technology
Nr. Standard reference Title Information technology - Open Systems Interconnection - The 1 ISO/IEC 9594-1:2017 Directory - Part 1: Overview of concepts, models and services Information technology - Open Systems Interconnection - The 2 ISO/IEC 9594-2:2017 Directory - Part 2: Models Information technology - Open Systems Interconnection - The 3 ISO/IEC 9594-3:2017 Directory - Part 3: Abstract service definition Information technology - Open Systems Interconnection - The 4 ISO/IEC 9594-4:2017 Directory - Part 4: Procedures for distributed operation Information technology - Open Systems Interconnection - The 5 ISO/IEC 9594-5:2017 Directory - Part 5: Protocol specifications Information technology - Open Systems Interconnection - The 6 ISO/IEC 9594-6:2017 Directory - Part 6: Selected attribute types Information technology - Open Systems Interconnection - The 7 ISO/IEC 9594-7:2017 Directory - Part 7: Selected object classes Information technology - Open Systems Interconnection - The 8 ISO/IEC 9594-8:2017 Directory - Part 8: Public-key and attribute certificate frameworks Information technology - Open Systems Interconnection - The 9 ISO/IEC 9594-9:2017 Directory - Part 9: Replication Paper and board - Determination of CIE whiteness, D65/10 10 ISO 11475:2017 degrees (outdoor daylight) Pulp, paper and board - Determination of total chlorine and 11 ISO 11480:2017 organically bound chlorine Non-alloy steel wire rod for conversion to wire - Part 1: General 12 ISO 16120-1:2017 requirements Non-alloy steel wire rod for conversion to wire - Part 4: Specific -

Progetto Definitivo
Rev. N. Data Contr. Approvazione 01 Agosto 2020 SDG SDG DISINQUINAMENTO FIUME PESCARA POTENZIAMENTO DEL SISTEMA DEPURATIVO COMUNE DI PESCARA NUOVO PARCO DEPURATIVO INTERVENTO 1 Realizzazione vasche di prima pioggia e disinfezione presso sollevamento B0 (Madonnina) PROGETTO DEFINITIVO GRUPPO DI PROGETTAZIONE: R.U.P.: Dott. Ing. Bartolomeo DI GIOVANNI Dott. Ing. Lorenzo LIVELLO Dott. Ing. Sante DI GIUSEPPE C&S Di Giuseppe Ingegneri Associati s.r.l. Geom. Tino DI PIETRANTONIO COLLABORAZIONI ERSI ABRUZZO: ARCHEOLOGIA: Dott. Luca CHERSTICH GEOLOGIA : Dott. Eustachio PIETROMARTIRE AMBIENTE : Dott. Nicola TAVANO Capitolato speciale d'appalto Elaborato Codice elaborato Scala 8.5 738PD08050000_01 - A.C.A. S.p.a. Agenzia Comprensoriale Acquedottistica S.p.a. CAPITOLATO SPECIALE D'APPALTO Progettazione esecutiva e realizzazione Parte amministrativa OGGETTO: Disinquinamento del fiume Pescara. PARTE D'OPERA: Parte Amministrativa COMMITTENTE: A.C.A. S.p.a. Codice CUP: ___________ Codice CIG: ___________ IL TECNICO C&S DI GIUSEPPE Ing. Ass. Srl pag.1 CAPITOLO 1 OGGETTO, FORMA E AMMONTARE DELL'APPALTO - AFFIDAMENTO E CONTRATTO - VARIAZIONI DELLE OPERE Art 1.1 OGGETTO DELL'APPALTO L'appalto ha per oggetto il servizio di progettazione esecutiva e l'esecuzione di tutte le opere e provviste occorrenti per eseguire e dare completamente ultimati i lavori relativi al "Disinquinamento del fiume Pescara. Potenziamento del sistema depurativo Comune di Pescara. Nuovo Parco depurativo - Intervento 1: Realizzazione vasche di prima pioggia e disinfezione presso sollevamento B0 (Madonnina)". Ai sensi dell'articolo 59 comma 1-bis del d. lgs. 50/2016 e s.m.i. sono compresi nell'appalto la progettazione esecutiva ed i lavori, le prestazioni, le forniture e le provviste necessarie per dare il lavoro completamente compiuto, secondo le condizioni stabilite dal presente capitolato speciale d'appalto, con le caratteristiche tecniche, qualitative e quantitative previste dal progetto definitivo dell'opera e relativi allegati dei quali l'Affidatario dichiara di aver preso completa ed esatta conoscenza. -

Standardiseringsprosjekter Og Nye Standarder
Annonseringsdato: 2017-10-25 Listenummer: 20/2017 Standardiseringsprosjekter og nye standarder Listenummer: 20/2017 Side: 1 av 136 01 Generelt. Terminologi. Standardisering. Dokumentasjon 01 Generelt. Terminologi. Standardisering. Dokumentasjon Standardforslag til høring - europeiske (CEN) prEN 1748-2-1 Glass in Building - Special basic products - Glass ceramics - Part 2-1: Definitions and general physical and mechanical properties Språk: en Kommentarfrist: 2017.11.02 prEN 12944-3 Fertilizers and liming materials - Vocabulary - Part 3: Terms relating to liming materials Språk: en Kommentarfrist: 2017.12.28 prEN 13279-1 Gypsum binders and gypsum plasters - Part 1: Definitions and requirements Språk: en Kommentarfrist: 2017.11.02 prEN 14564 Tanks for the transport of dangerous goods - Terminology Språk: en Kommentarfrist: 2017.12.28 prEN 17161 Design for All - Accessibility following a Design for All approach in products, goods and services - Extending the range of users Språk: en Kommentarfrist: 2017.11.02 prEN ISO 4007 Personal protective equipment - Eye and face protection - Vocabulary (ISO/DIS 4007:2017) Språk: en Kommentarfrist: 2017.10.27 prEN ISO 8384 Ships and marine technology - Dredgers - Vocabulary (ISO/DIS 8384:2017) Språk: en Kommentarfrist: 2017.11.15 prEN ISO 11139 Sterilization of health care products - Vocabulary - Terms used in sterilization and related equipment and process standards (ISO/DIS 11139:2017) Språk: en Kommentarfrist: 2017.11.02 prEN ISO 14880-1 Optics and photonics - Microlens arrays - Part 1: Vocabulary (ISO/DIS 14880-1:2017) Språk: en Kommentarfrist: 2017.12.20 Standardforslag til høring - internasjonale (ISO) ISO/DIS 8384 Ships and marine technology - Dredgers - Vocabulary Språk: en Kommentarfrist: 2017.11.14 Listenummer: 20/2017 Side: 2 av 136 01 Generelt. -

Documentos Normativos
Publicação oficial do IPQ, enquanto Organismo Nacional de Normalização lista mensal . março 2014 A presente publicação tem por objetivo: - A divulgação das Normas Portuguesas recentemente editadas e anuladas, bem como das Normas Europeias adotadas como Normas Portuguesas, e das Normas Europeias e Internacionais publicadas e já disponíveis. - A divulgação pública dos projetos de Normas Portuguesas, Europeias e Internacionais, com vista à obtenção durante o período de inquérito público dos pontos de vista e contribuições nacionais que possam ser considerados na sequente elaboração, aprovação e homologação das Normas. - A divulgação de propostas de novos trabalhos de normalização europeia e internacional, para que se possa obter, durante o período de inquérito público, os pontos de vista e as contribuições nacionais. - A divulgação da edição e anulação de outros documentos normativos. ÍNDICE 1. Normas Portuguesas 1.1 Normas Portuguesas publicadas …………………………………………………………………………. 3 1.2 Normas Portuguesas anuladas …………………………………………………………………………… 5 1.3 Normas Portuguesas em re-exame ……………………………………………………………………… 6 1.4 Normas Europeias adotadas ……………………………………………………………………………… 7 2. Normas Europeias Publicadas 2.1 CEN …………………………………………………………………………………………………………….. 12 2.2 CENELEC ………………………………………………………………………………………………………. 15 2.3 CEN/CENELEC ……………………………………………………………………………………………….. 17 2.4 ETSI ……………………………………………………………………………………………………………… 18 3. Normas Internacionais publicadas IEC …………………………………………………………………………………………………………………… 19 ISO …………………………………………………………………………………………………………………..