First Synthesis of Important Secondary Oxidative Metabolites of Morphine and Codeine with the Michael Addition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alternative Formats If You Require This Document in an Alternative Format, Please Contact: [email protected]
University of Bath PHD The extraction and chemistry of the metabolites of Mimosa tenuiflora and Papaver somniferum Ninan, Aleyamma Award date: 1990 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 23. Sep. 2021 THE EXTRACTION AND CHEMISTRY OF THE METABOLITES OF MIMOSA TENUIFLORA AND PAP AVER SOMNIFERUM. submitted by ALEYAMMA NINAN for the degree of Doctor of Philosophy of the University of Bath 1990 Attention is drawn to the fact that the copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without prior consent of the author. -

The Metamorphosis of Hydromorphone Gary M
Pharmacy PersPective The metamorphosis of hydromorphone Gary M. Reisfield, MD George R. Wilson, MD introduction “That’s it. The Mayo Clinic at Rochester devel - oped it, the word and the drug, for it means a Hydromorphone hydrochloride, one of the oldest of drug, a pain relieving drug, five times as potent the extant opioid analgesics, has been in clinical use for as morphine, as harmless as water and with no more than 70 years. Its use by the oral route in chronic habit forming qualities. pain and hospice/palliative medicine settings has been limited, however, largely owing to its relatively short “The medical journals say it is particularly useful duration of action. With the recent US Food and Drug in the operation of cases where other drugs Administration (FDA) approval of a once-daily extended- seem to offer no relief from pain. Unlike mor - release formulation of the drug (Palladone, Purdue phine, there are no pleasurable sensations to its Pharma LP, Stamford, CT), hydromorphone joins mor - use, however, and if the doctors reckon correctly phine, oxycodone, and fentanyl as the only extended- its use may go far toward curing addicts of the release opioids available on the United States market. morphine habit.” Here, we review the history, pharmacokinetics, and other relevant issues concerning this invaluable opioid, and Montgomery (AL) Advertiser , Dec. 18, 1932 also discuss the role of the new formulation in the man - agement of chronic pain. From 1929 to 1939, the National Research Council’s Committee on Drug Addiction conducted exhaustive history research on the morphine molecule and its analogs, pro - ducing more than 150 semisynthetic and more than 300 Hydromorphone [also Dilaudid (Knoll Laboratories, synthetic compounds, of which more than 30 were tested Mount Olive, NJ), dihydromorphinone, dihydromorphe - clinically. -

Morphinone Reductase for the Preparation Of
Europäisches Patentamt *EP000649465B1* (19) European Patent Office Office européen des brevets (11) EP 0 649 465 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C12N 9/02, C12P 17/18, of the grant of the patent: C12Q 1/32 24.07.2002 Bulletin 2002/30 (86) International application number: (21) Application number: 93913236.1 PCT/GB93/01129 (22) Date of filing: 28.05.1993 (87) International publication number: WO 94/00565 (06.01.1994 Gazette 1994/02) (54) MORPHINONE REDUCTASE FOR THE PREPARATION OF HYDROMORPHONE AND HYDROCODONE MORPHINONE REDUKTASE ZUR HERSTELLUNG VON HYDROMORPHONE UND HYDROCODONE MORPHINONE REDUCTASE DESTINEE A LA PREPARATION D’HYDROMORPHONE ET D’HYDROCODONE (84) Designated Contracting States: (74) Representative: Perry, Robert Edward AT BE CH DE DK ES FR GB IE IT LI NL PT SE GILL JENNINGS & EVERY Broadgate House (30) Priority: 25.06.1992 GB 9213524 7 Eldon Street London EC2M 7LH (GB) (43) Date of publication of application: 26.04.1995 Bulletin 1995/17 (56) References cited: WO-A-90/13634 (73) Proprietor: MACFARLAN SMITH LIMITED Edinburgh EH11 2QA (GB) • THE BIOCHEMICAL JOURNAL vol. 274, no. 3, 15 March 1991, pages 875 - 880 NEIL C. BRUCE ET (72) Inventors: AL. ’Microbial degradation of the morphine • HAILES, Anne, Maria 59 Lower Road alkaloids. Purification and characterization of Kent DA8 1AY (GB) morphine dehydrogenase from Pseudomonas • FRENCH, Christopher, Edward putida M10’ Palmerston North (NZ) • BRUCE, Neil, Charles Cambridge CB2 1ND (GB) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Effect of Anticancer Agents on Codeinone-Induced Apoptosis in Human Cancer Cell Lines
ANTICANCER RESEARCH 25: 4037-4042 (2005) Effect of Anticancer Agents on Codeinone-induced Apoptosis in Human Cancer Cell Lines RISA TAKEUCHI1,2, HIROSHI HOSHIJIMA1,2, NORIKO ONUKI1,2, HIROSHI NAGASAKA1, SHAHEAD ALI CHOWDHURY3, MASAMI KAWASE4 and HIROSHI SAKAGAMI2 1Division of Anesthesiology, Department of Comprehensive Medical Sciences, 2Division of Pharmacology, Department of Diagnostic and Therapeutic Sciences, and 3MPL (Meikai Phamaco-Medical Laboratory), Meikai University School of Dentistry, Sakado, Saitama 350-0283; 4Faculty of Phamaceutical Sciences, Josai University, Sakado, Saitama 350-0295, Japan Abstract. The possible apoptosis-inducing activity of Opioids have been given to patients with cancer pain, codeinone, an oxidative metabolite of codeine, without or according to the WHO ladder against pain (4). According with anticancer drugs, was investigated. Codeinone induced to reports of Ventafridda et al., pain occurs in more than internucleosomal DNA fragmentation in human 50% of cancer patients, and the administration of opioids promyelocytic leukemia cells (HL-60), but not in human manifests significantly higher therapeutic effects against squamous cell carcinoma cells (HSC-2). Codeinone dose- cancer pain, compared with non-narcotics (5, 6). Although dependently activated caspase-3 in both of these cells, but to opioids have been used for patients in clinical situations, the a much lesser extent than that attained by actinomycin D. anticancer action of opioids has not been well investigated. This property of codeinone was similar to what we have Both opioids and anticancer drugs have been simultaneously found previously in ·,‚-unsaturated ketones. Codeinone did given for pain in cancer patients. However, there has been not activate caspase-8 or caspase-9 in these cells. -

Microbial Degradation of the Morphine Alkaloids Purification and Characterization of Morphine Dehydrogenase from Pseudomonas Putida M10
Biochem. J. (1991) 274, 875-880 (Printed in Great Britain) 875 Microbial degradation of the morphine alkaloids Purification and characterization of morphine dehydrogenase from Pseudomonas putida M10 Neil C. BRUCE,* Clare J. WILMOT, Keith N. JORDAN, Lauren D. Gray STEPHENS and Christopher R. LOWE Institute of Biotechnology, University of Cambridge, Tennis Court Road, Cambridge CB2 1QT, U.K. The NADP+-dependent morphine dehydrogenase that catalyses the oxidation of morphine to morphinone was detected in glucose-grown cells of Pseudomonas putida M 10. A rapid and reliable purification procedure involving two consecutive affinity chromatography steps on immobilized dyes was developed for purifying the enzyme 1216-fold to electrophoretic homogeneity from P. putida M 10. Morphine dehydrogenase was found to be a monomer of Mr 32000 and highly specific with regard to substrates, oxidizing only the C-6 hydroxy group of morphine and codeine. The pH optimum of morphine dehydrogenase was 9.5, and at pH 6.5 in the presence of NADPH the enzyme catalyses the reduction of codeinone to codeine. The Km values for morphine and codeine were 0.46 mm and 0.044 mm respectively. The enzyme was inhibited by thiol-blocking reagents and the metal-complexing reagents 1, 10-phenanthroline and 2,2'-dipyridyl, suggesting that a metal centre may be necessary for activity of the enzyme. INTRODUCTION EXPERIMENTAL The morphine alkaloids have attracted considerable attention Materials owing to their analgaesic properties and, consequently, much Mimetic Orange 3 A6XL and Mimetic Red A6XL were effort in the past has been directed at the production of new obtained from Affinity Chromatography Ltd., Freeport, morphine alkaloids by micro-organisms (lizuka et al., 1960, Ballasalla, Isle of Man, U.K. -
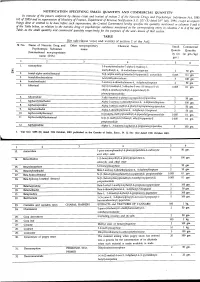
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

0 Cover Part Two\374
PART TWO DEUXIÈME PARTIE SEGUNDA PARTE ﺍﳉﺰﺀ ﺍﻟﺜﺎﱐ 第二部分 ЧАСТЬ ВТΟΡАЯ - (...), [...] - (-)-3-hidroxi-N- (-)-(2R)-N-méthyl-1- fenacilmorfinán → Levophenacyl-morphan phénylpropan-2-amine → Levometamfetamine (-)-3-hidroxi-N- (-)-(3S,6R)-6- metilmorfinán → Levorphanol (dimethylamino)-4,4- (-)-3-hydroksymorfinan → Norlevorphanol diphenyl-3-heptanol → Betamethadol (-)-3-hydroksy-N- (-)-(3S,6R)-6- fenacylmorfinan → Levophenacyl-morphan (dimethylamino)-4,4- (-)-3-hydroksy-N- diphenyl-3-heptanol metylmorfinan → Levorphanol acetate (ester) → Betacetylmethadol (-)-3-hydroxymorphinan → Norlevorphanol (-)-(5R)-4,5-epoxy-3- (-)-3-hydroxy-N- methoxy-9α-methyl methylmorphinan → Levorphanol → Thebacon morphin-6-en-6-yl acetate (-)-3-hydroxynormorphinan → Norlevorphanol (-)-(5R,6S)-3-benzyloxy-4,5- (-)-3-hydroxy-N- epoxy-9a-methylmorphin- phenacylmorphinan → Levophenacyl-morphan 7-en-6-yl myristate → Myrophine (-)-3-hydroxytropane-2- (-)-(5R,6S)-4,5-epoxy carboxylat → Ecgonine morphin-7-en-3,6-diol → Normorphine (-)-3-idrossi-N- (-)-(5R,6S,14S)-4,5-epoxy- metilmorfinano → Levorphanol 14-hydroxy-3-methoxy- (-)-3-methoxy-N- 9a-methylmorphinan-6- methylmorphinan → Levomethorphan one → Oxycodone (-)-3-metil-2,2-difenil-4- (-)-(R)-4,5-epoxy-3- morfolinbutirilpirrolidina → Levomoramide methoxy-9a-methyl (-)-3-metoksy-N- morphinan-6-one → Hydrocodone metylmorfinan → Levomethorphan (-)-(R)-6-(dimethylamino)- (-)-3-metossi-N-metil- 4,4-diphenyl-3-heptanone → l-methadone morfinano → Levomethorphan (-)-(R)-N,α-dimethyl (-)-3-metoxi-N- phenethylamine → Levometamfetamine -

Ep 2398808 B1
(19) TZZ ¥Z_T (11) EP 2 398 808 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 489/08 (2006.01) 20.11.2013 Bulletin 2013/47 (86) International application number: (21) Application number: 10704301.0 PCT/US2010/024372 (22) Date of filing: 17.02.2010 (87) International publication number: WO 2010/096416 (26.08.2010 Gazette 2010/34) (54) Process for the reductive alkylation of normorphinans VERFAHREN ZUR REDUKTIVEN ALKYLIERUNG VON NORMORPHINANEN Procédé d’alkylation réductrice des normorphinanes (84) Designated Contracting States: • WOODS, Sharon AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Florissant HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL Missouri 63034 (US) PT RO SE SI SK SM TR (74) Representative: Beckmann, Claus (30) Priority: 17.02.2009 US 153021 P Kraus & Weisert Patent- und Rechtsanwälte (43) Date of publication of application: Thomas-Wimmer-Ring 15 28.12.2011 Bulletin 2011/52 80539 München (DE) (73) Proprietor: Mallinckrodt LLC (56) References cited: Hazelwood, MO 63042 (US) WO-A1-2006/035195 WO-A1-2009/012005 (72) Inventors: • HUDSON, Edmund, C. Clayton Missouri 63105 (US) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. Notice of opposition shall not be deemed to have been filed until the opposition fee has been paid. -

Back Matter (PDF)
The Editor of the Journal of Pharmacology & Experimental Therapeutics wishes to express appreciation to the following colleagues who acted as Guest Editors for Specific Fields in 1987. Martin W. Adler Frank R. Goodman Elaine Sanders-Bush Lewis Aronow Robert Z. Gussin Lewis E. Seiden C. Paul Bianchi Conan Kornetsky Theodore J. Torphy Allan H. Conney Donald J. Jenden John L. Skosey Theodore J. Cicero Robert M. Joy Larry G. Stark Charles R. Craig Louis Lemberger Norman Weiner Gerard L. Gebber Benedict R. Lucchesi Wallace D. Winters James W. Gibb Donald V. Priola Dixon M. Woodbury Dora B. Goldstein Additionally, sincere thanks are due to 724 of our colleagues not on the Editorial Advisory Board who reviewed articles for the Journal in 1987. Their contributions are acknowledged individually in the Annual Report of The Journal to The Board of Publications Trustees of The American Society for Pharmacology and Experimental Therapeutics. INDEX Volume 245, April-June, 1988 A23187, effect ofextracellularcalcium omis- classification, ligand affmity and mo- -induced intoxication, effects of Ro15- sion, hepatocytes (rats), 493 lecular mass (bovine), 1060 4513 (rats), 880 Abhold, R. H. and Harding, J. W.: Metabo- imidazoline analogs, aorta (rats), 793 Mi, S. F., see Schulze, G. E., 178 lism of angiotensins II and III by inotropic positive effect, inositol tris- Alkondon, M., Rao, K. S. and Albuquerque, membrane-bound peptidases from phosphate increase in heart (rats), E. X.: Acetylcholinesterase reactiva- rat brain, 171 327 tom modify the functional properties Abiko, Y., see Hara, Y., 305 prazosin and indoramin, coronary of the nicotinic acetylcholine recep- Acetaminophen blood flow (dogs), 232 tor ion channel, 543 diminuition of hepatotoxicity, ethanol alpha-2 Allbee, W. -

The Identification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae
Washington University in St. Louis Washington University Open Scholarship Arts & Sciences Electronic Theses and Dissertations Arts & Sciences Winter 12-15-2015 The deI ntification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae Matthew .B Kilgore Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/art_sci_etds Recommended Citation Kilgore, Matthew B., "The deI ntification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae" (2015). Arts & Sciences Electronic Theses and Dissertations. 657. https://openscholarship.wustl.edu/art_sci_etds/657 This Dissertation is brought to you for free and open access by the Arts & Sciences at Washington University Open Scholarship. It has been accepted for inclusion in Arts & Sciences Electronic Theses and Dissertations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS Division of Biology and Biomedical Sciences Plant Biology Dissertation Examination Committee: Toni Kutchan, Chair Elizabeth Haswell Jeffrey Henderson Joseph Jez Barbara Kunkel Todd Mockler The Identification of Alkaloid Pathway Genes from Non-Model Plant Species in the Amaryllidaceae by Matthew Benjamin Kilgore A dissertation presented to the Graduate School of Arts & Sciences of Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2015 St. Louis, Missouri -

(12) United States Patent (10) Patent No.: US 6,469,170 B1 Chiu Et Al
USOO646917OB1 (12) United States Patent (10) Patent No.: US 6,469,170 B1 Chiu et al. (45) Date of Patent: Oct. 22, 2002 (54) METHOD FOR PREPARING OXYCODONE (58) Field of Search ............................................ 546/45 (75) Inventors: Fang-Ting Chiu, Chesterfield, VA (56) References Cited (US); Young S. Lo, Chester, VA (US) FOREIGN PATENT DOCUMENTS (73) Assignee: Boehringer Ingelheim Chemicals, Inc., Petersburg, VA (US) WO 9902529 * 1/1999 (*) Notice: Subject to any disclaimer, the term of this * cited by examiner patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. Primary Examiner Alan L. Rotman ASSistant Examiner Binta Robinson (21) Appl. No.: 10/152,140 (74) Attorney, Agent, or Firm-Robert P. Raymond; Mary-Ellen M. Devlin; Alan R. Stempel (22)22) FileFilled: Maway Zl,21, 2002 (57) ABSTRACT Related U.S. Application Data A method for the preparation of oxycodone, and Salts (62) Division of application No. 09/793,024, filed on Feb. 26 thereof, from codeine comprising oxidation of codeine to 2001, now Pat. No. 6.403.798, which is a division of codeinone, formation of an dienolsilyl ether congener of application No. 09/667.997, filed on Sep. 22, 2000, now Pat. codeinone in Strong amine base, oxidation of the dienolsilyl No. 6,262,266, which is a division of application No. ether congener using peracetic acid, and hydrogenation of 09/419,429, filed on Oct. 15, 1999, now Pat. No. 6,177.567. the resulting 14-hydroxycodeinone product. (51) Int. Cl." .............................................. C07D 471/00 (52) U.S. Cl. ......................................................... 546/45 8 Claims, No Drawings US 6,469,170 B1 1 METHOD FOR PREPARING OXYCODONE BACKGROUND OF THE INVENTION 1. -

Recent Advances in the Chemistry of Oripavine and Its Derivatives
Advances in Bioscience and Biotechnology, 2014, 5, 704-717 Published Online July 2014 in SciRes. http://www.scirp.org/journal/abb http://dx.doi.org/10.4236/abb.2014.58084 Recent Advances in the Chemistry of Oripavine and Its Derivatives Sandor Hosztafi Institute of Pharmaceutical Chemistry, Semmelweis University, Budapest, Hungary Email: [email protected] Received 2 May 2014; revised 16 June 2014; accepted 12 July 2014 Copyright © 2014 by author and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Oripavine is the major alkaloid of Papaver orientale. It is an important intermediate in the bio- synthesis of morphine alkaloids. Recently, new Papaver somniferum strains have been developed which accumulate thebaine and oripavine, but not morphine and codeine. Therefore, the chemi- stry of oripavine has been studied intensively to synthesize opioid pharmaceuticals such as oxy- morphone, naloxone and buprenorphine. Keywords Papaver orientale, Papaver somniferum, Oripavine, Thebaine, Top1 Poppy 1. Introduction Thebaine an alkaloid present in 0.2% - 0.8% in opium and a major constituent (90% of total alkaloid content) in Papaver bracteatum (which is morphine free), possesses little utility medically for two reasons: (a) its lack of the depressant and analgesic properties common to other morphine alkaloids and (b) its expression of extreme toxicity and CNS stimulation. Oripavine is also a minor alkaloid of the opium poppy, but it is the main alkaloid of the oriental poppy Papaver orientale, which is a perennial flowering plant. The development of the top1 poppy was an important finding for the opium industry in Australia, because this poppy strain produces thebaine and oripavine, but not morphine or codeine.