Supplementary Material Table S1. BLAST Database with the Downloaded EST Sequences Involved in the Wood Production Process. in Pa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Populusspp. Family: Salicaceae Aspen
Populus spp. Family: Salicaceae Aspen Aspen (the genus Populus) is composed of 35 species which contain the cottonwoods and poplars. Species in this group are native to Eurasia/north Africa [25], Central America [2] and North America [8]. All species look alike microscopically. The word populus is the classical Latin name for the poplar tree. Populus grandidentata-American aspen, aspen, bigtooth aspen, Canadian poplar, large poplar, largetooth aspen, large-toothed poplar, poplar, white poplar Populus tremuloides-American aspen, American poplar, aspen, aspen poplar, golden aspen, golden trembling aspen, leaf aspen, mountain aspen, poplar, popple, quaking asp, quaking aspen, quiver-leaf, trembling aspen, trembling poplar, Vancouver aspen, white poplar Distribution Quaking aspen ranges from Alaska through Canada and into the northeastern and western United States. In North America, it occurs as far south as central Mexico at elevations where moisture is adequate and summers are sufficiently cool. The more restricted range of bigtooth aspen includes southern Canada and the northern United States, from the Atlantic coast west to the prairie. The Tree Aspens can reproduce sexually, yielding seeds, or asexually, producing suckers (clones) from their root system. In some cases, a stand could then be composed of only one individual, genetically, and could be many years old and cover 100 acres (40 hectares) or more. Most aspen stands are a mosaic of several clones. Aspen can reach heights of 120 ft (48 m), with a diameter of 4 ft (1.6 m). Aspen trunks can be quite cylindrical, with little taper and few limbs for most of their length. They also can be very crooked or contorted, due to genetic variability. -

Checklist of Common Native Plants the Diversity of Acadia National Park Is Refl Ected in Its Plant Life; More Than 1,100 Plant Species Are Found Here
National Park Service Acadia U.S. Department of the Interior Acadia National Park Checklist of Common Native Plants The diversity of Acadia National Park is refl ected in its plant life; more than 1,100 plant species are found here. This checklist groups the park’s most common plants into the communities where they are typically found. The plant’s growth form is indicated by “t” for trees and “s” for shrubs. To identify unfamiliar plants, consult a fi eld guide or visit the Wild Gardens of Acadia at Sieur de Monts Spring, where more than 400 plants are labeled and displayed in their habitats. All plants within Acadia National Park are protected. Please help protect the park’s fragile beauty by leaving plants in the condition that you fi nd them. Deciduous Woods ash, white t Fraxinus americana maple, mountain t Acer spicatum aspen, big-toothed t Populus grandidentata maple, red t Acer rubrum aspen, trembling t Populus tremuloides maple, striped t Acer pensylvanicum aster, large-leaved Aster macrophyllus maple, sugar t Acer saccharum beech, American t Fagus grandifolia mayfl ower, Canada Maianthemum canadense birch, paper t Betula papyrifera oak, red t Quercus rubra birch, yellow t Betula alleghaniesis pine, white t Pinus strobus blueberry, low sweet s Vaccinium angustifolium pyrola, round-leaved Pyrola americana bunchberry Cornus canadensis sarsaparilla, wild Aralia nudicaulis bush-honeysuckle s Diervilla lonicera saxifrage, early Saxifraga virginiensis cherry, pin t Prunus pensylvanica shadbush or serviceberry s,t Amelanchier spp. cherry, choke t Prunus virginiana Solomon’s seal, false Maianthemum racemosum elder, red-berried or s Sambucus racemosa ssp. -

Poplars and Willows: Trees for Society and the Environment / Edited by J.G
Poplars and Willows Trees for Society and the Environment This volume is respectfully dedicated to the memory of Victor Steenackers. Vic, as he was known to his friends, was born in Weelde, Belgium, in 1928. His life was devoted to his family – his wife, Joanna, his 9 children and his 23 grandchildren. His career was devoted to the study and improve- ment of poplars, particularly through poplar breeding. As Director of the Poplar Research Institute at Geraardsbergen, Belgium, he pursued a lifelong scientific interest in poplars and encouraged others to share his passion. As a member of the Executive Committee of the International Poplar Commission for many years, and as its Chair from 1988 to 2000, he was a much-loved mentor and powerful advocate, spreading scientific knowledge of poplars and willows worldwide throughout the many member countries of the IPC. This book is in many ways part of the legacy of Vic Steenackers, many of its contributing authors having learned from his guidance and dedication. Vic Steenackers passed away at Aalst, Belgium, in August 2010, but his work is carried on by others, including mem- bers of his family. Poplars and Willows Trees for Society and the Environment Edited by J.G. Isebrands Environmental Forestry Consultants LLC, New London, Wisconsin, USA and J. Richardson Poplar Council of Canada, Ottawa, Ontario, Canada Published by The Food and Agriculture Organization of the United Nations and CABI CABI is a trading name of CAB International CABI CABI Nosworthy Way 38 Chauncey Street Wallingford Suite 1002 Oxfordshire OX10 8DE Boston, MA 02111 UK USA Tel: +44 (0)1491 832111 Tel: +1 800 552 3083 (toll free) Fax: +44 (0)1491 833508 Tel: +1 (0)617 395 4051 E-mail: [email protected] E-mail: [email protected] Website: www.cabi.org © FAO, 2014 FAO encourages the use, reproduction and dissemination of material in this information product. -

Poplar Chap 1.Indd
Populus: A Premier Pioneer System for Plant Genomics 1 1 Populus: A Premier Pioneer System for Plant Genomics Stephen P. DiFazio,1,a,* Gancho T. Slavov 1,b and Chandrashekhar P. Joshi 2 ABSTRACT The genus Populus has emerged as one of the premier systems for studying multiple aspects of tree biology, combining diverse ecological characteristics, a suite of hybridization complexes in natural systems, an extensive toolbox of genetic and genomic tools, and biological characteristics that facilitate experimental manipulation. Here we review some of the salient biological characteristics that have made this genus such a popular object of study. We begin with the taxonomic status of Populus, which is now a subject of ongoing debate, though it is becoming increasingly clear that molecular phylogenies are accumulating. We also cover some of the life history traits that characterize the genus, including the pioneer habit, long-distance pollen and seed dispersal, and extensive vegetative propagation. In keeping with the focus of this book, we highlight the genetic diversity of the genus, including patterns of differentiation among populations, inbreeding, nucleotide diversity, and linkage disequilibrium for species from the major commercially- important sections of the genus. We conclude with an overview of the extent and rapid spread of global Populus culture, which is a testimony to the growing economic importance of this fascinating genus. Keywords: Populus, SNP, population structure, linkage disequilibrium, taxonomy, hybridization 1Department of Biology, West Virginia University, Morgantown, West Virginia 26506-6057, USA; ae-mail: [email protected] be-mail: [email protected] 2 School of Forest Resources and Environmental Science, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, USA; e-mail: [email protected] *Corresponding author 2 Genetics, Genomics and Breeding of Poplar 1.1 Introduction The genus Populus is full of contrasts and surprises, which combine to make it one of the most interesting and widely-studied model organisms. -

Abstract on the Aspen Association of Northern Michigan
Abstract on the Aspen Association of Northern Michigan Prepared by: Christopher R . Weber Joshua G . Cohen Michael A . Kost Michigan Natural Features Inventory P.O. Box 30444 Lansing, MI 48909-7944 For: Michigan Department of Natural Resources Forest, Minerals, and Fire Management Division Wildlife Division September 30, 2006 Report Number 2006-16 Cover Photograph: Young aspen clones in the eastern Upper Peninsula, Michigan. All photos by Christopher R. Weber unless otherwise noted. Overview Range This document provides a brief discussion of the aspen Trembling aspen has the most extensive range of any association of Michigan, detailing this system’s North American tree (Barnes et al. 1998). Its native landscape and historical context, range, ecological range covers a large swath from the Atlantic Ocean to processes, characteristic vegetation and fauna, and the Pacific Ocean, spreading from its southern limit in threatened and endangered species. In addition, northern Illinois, Indiana, Ohio, and Pennsylvania north potential options and strategies are suggested for to the Hudson Bay. Trembling aspen’s range reaches enhancing biodiversity of managed aspen associations into the northern and southern Rocky Mountains, and and for restoring these systems to later successional all the way west through Canada and east to forest types. Newfoundland and Labrador (Perala 1990). Throughout the south and west Rocky Mountains, trembling aspen is found as a late-successional species, having long-lived clones, sometimes with Introduction thousands of stems that expand over large acreages The aspen association occurs throughout the Great (Barnes et al. 1998). Aspen has been found to Lakes region as a disturbance dependent vegetation compete with prairies in the Canadian west (Bird assemblage. -

A Field Guide to Selected Trees and Shrubs of the University of Massachusetts Dartmouth Campus
A Field Guide to Selected Trees and Shrubs of the University of Massachusetts Dartmouth Campus Written and Illustrated by: Richard H. Uva Class of 1991 Department of Biology Univeristy of Massachusetts Dartmouth North Dartmouth, MA Edited by: James R. Sears Professor of Biology University of Massachusetts Dartmouth Converted to Web Format by: Sustainability Studies Seminar Class Spring 2012 University of Massachusetts Dartmouth 1 Table of Contents RED MAPLE Ȃ ACER RUBRUM ............................................................................................................. 4 YELLOW BIRCH - BETULA LUTEA ...................................................................................................... 6 GRAY BIRCH - BETULA POPULIFOLIA .............................................................................................. 8 SWEET PEPPERBUSH - CLETHRA ALNIFOLIA .............................................................................. 10 SWEETFERN - COMPTONIA PEREGRINE ........................................................................................ 11 FLOWERING DOGWOOD - CORNUS FLORIDA .............................................................................. 13 AMERICAN BEECH - FAGUS GRANDIFOLIA ................................................................................... 15 WITCH-HAZEL - HAMAMELIS VIRGINIANA ................................................................................... 17 INKBERRY - ILEX GLABRA ................................................................................................................ -

Trees, Shrubs, and Perennials That Intrigue Me (Gymnosperms First
Big-picture, evolutionary view of trees and shrubs (and a few of my favorite herbaceous perennials), ver. 2007-11-04 Descriptions of the trees and shrubs taken (stolen!!!) from online sources, from my own observations in and around Greenwood Lake, NY, and from these books: • Dirr’s Hardy Trees and Shrubs, Michael A. Dirr, Timber Press, © 1997 • Trees of North America (Golden field guide), C. Frank Brockman, St. Martin’s Press, © 2001 • Smithsonian Handbooks, Trees, Allen J. Coombes, Dorling Kindersley, © 2002 • Native Trees for North American Landscapes, Guy Sternberg with Jim Wilson, Timber Press, © 2004 • Complete Trees, Shrubs, and Hedges, Jacqueline Hériteau, © 2006 They are generally listed from most ancient to most recently evolved. (I’m not sure if this is true for the rosids and asterids, starting on page 30. I just listed them in the same order as Angiosperm Phylogeny Group II.) This document started out as my personal landscaping plan and morphed into something almost unwieldy and phantasmagorical. Key to symbols and colored text: Checkboxes indicate species and/or cultivars that I want. Checkmarks indicate those that I have (or that one of my neighbors has). Text in blue indicates shrub or hedge. (Unfinished task – there is no text in blue other than this text right here.) Text in red indicates that the species or cultivar is undesirable: • Out of range climatically (either wrong zone, or won’t do well because of differences in moisture or seasons, even though it is in the “right” zone). • Will grow too tall or wide and simply won’t fit well on my property. -

Vegetation Classification and Mapping Project Report
U.S. Geological Survey-National Park Service Vegetation Mapping Program Acadia National Park, Maine Project Report Revised Edition – October 2003 Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U. S. Department of the Interior, U. S. Geological Survey. USGS-NPS Vegetation Mapping Program Acadia National Park U.S. Geological Survey-National Park Service Vegetation Mapping Program Acadia National Park, Maine Sara Lubinski and Kevin Hop U.S. Geological Survey Upper Midwest Environmental Sciences Center and Susan Gawler Maine Natural Areas Program This report produced by U.S. Department of the Interior U.S. Geological Survey Upper Midwest Environmental Sciences Center 2630 Fanta Reed Road La Crosse, Wisconsin 54603 and Maine Natural Areas Program Department of Conservation 159 Hospital Street 93 State House Station Augusta, Maine 04333-0093 In conjunction with Mike Story (NPS Vegetation Mapping Coordinator) NPS, Natural Resources Information Division, Inventory and Monitoring Program Karl Brown (USGS Vegetation Mapping Coordinator) USGS, Center for Biological Informatics and Revised Edition - October 2003 USGS-NPS Vegetation Mapping Program Acadia National Park Contacts U.S. Department of Interior United States Geological Survey - Biological Resources Division Website: http://www.usgs.gov U.S. Geological Survey Center for Biological Informatics P.O. Box 25046 Building 810, Room 8000, MS-302 Denver Federal Center Denver, Colorado 80225-0046 Website: http://biology.usgs.gov/cbi Karl Brown USGS Program Coordinator - USGS-NPS Vegetation Mapping Program Phone: (303) 202-4240 E-mail: [email protected] Susan Stitt USGS Remote Sensing and Geospatial Technologies Specialist USGS-NPS Vegetation Mapping Program Phone: (303) 202-4234 E-mail: [email protected] Kevin Hop Principal Investigator U.S. -

Variation and Integration of Ecophysiological Traits Across Scales in Tropical and Temperate Trees: Patterns, Drivers and Consequences
Variation and Integration of Ecophysiological Traits across Scales in Tropical and Temperate Trees: Patterns, Drivers and Consequences Item Type text; Electronic Dissertation Authors Messier, Julie Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 10/10/2021 23:02:47 Link to Item http://hdl.handle.net/10150/594556 VARIATION AND INTEGRATION OF ECOPHYSIOLOGICAL TRAITS ACROSS SCALES IN TROPICAL AND TEMPERATE TREES: PATTERNS, DRIVERS AND CONSEQUENCES by Julie Messier __________________________ Copyright © Julie Messier 2015 A Dissertation Submitted to the Faculty of the DEPARTMENT OF ECOLOGY AND EVOLUTIONARY BIOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2015 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Julie Messier, titled “Variation and integration of ecophysiological traits across scales in tropical and temperate trees: patterns, drivers and consequences”, and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy. _______________________________________________________________________ Date: -

Natural Hybridization Between Cultivated Poplars and Their Wild
Natural hybridization between cultivated poplars and their wild relatives: evidence and consequences for native poplar populations An Vanden Broeck, Marc Villar, Erik van Bockstaele, Jos Vanslycken To cite this version: An Vanden Broeck, Marc Villar, Erik van Bockstaele, Jos Vanslycken. Natural hybridization between cultivated poplars and their wild relatives: evidence and consequences for native poplar populations. Annals of Forest Science, Springer Nature (since 2011)/EDP Science (until 2010), 2005, 62 (7), pp.601- 613. hal-00883933 HAL Id: hal-00883933 https://hal.archives-ouvertes.fr/hal-00883933 Submitted on 1 Jan 2005 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Ann. For. Sci. 62 (2005) 601–613 601 © INRA, EDP Sciences, 2005 DOI: 10.1051/forest:2005072 Review Natural hybridization between cultivated poplars and their wild relatives: evidence and consequences for native poplar populations An VANDEN BROECKa*, Marc VILLARb, Erik VAN BOCKSTAELEc, Jos VAN SLYCKENa a Institute for Forestry and Game Management (IBW), Research Station of the Flemish -
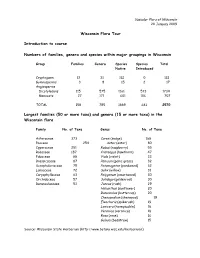
Wisconsin Flora Tour Introduction to Course Numbers of Families, Genera
Vascular Flora of Wisconsin 20 January 2009 Wisconsin Flora Tour Introduction to course Numbers of families, genera and species within major groupings in Wisconsin Group Families Genera Species Species Total Native Introduced Cryptogams 13 31 112 0 112 Gymnosperms 3 8 15 2 17 Angiosperms Dicotyledons 115 575 1161 573 1734 Monocots 27 171 601 106 707 TOTAL 158 785 1889 681 2570 Largest families (50 or more taxa) and genera (15 or more taxa) in the Wisconsin flora Family No. of Taxa Genus No. of Taxa Asteraceae 373 Carex (sedge) 168 Poaceae 254 Aster (aster) 80 Cyperaceae 251 Rubus (raspberry) 55 Rosaceae 187 Crateagus (hawthorn) 47 Fabaceae 88 Viola (violet) 33 Brassicaceae 87 Panicum (panic grass) 32 Scrophulariaceae 75 Potamogeton (pondweed) 32 Lamiaceae 72 Salix (willow) 31 Caryophyllaceae 63 Polygonum (smartweed) 30 Orchidaceae 57 Solidago (goldenrod) 30 Ranunculaceaee 53 Juncus (rush) 29 Helianthus (sunflower) 20 Ranunculus (buttercup) 20 Chenopodium (chenopod) 19 Eleocharis (spikerush) 19 Lonicera (honeysuckle) 18 Veronica (veronica) 18 Rosa (rose) 16 Galium (bedstraw) 15 Source: Wisconsin State Herbarium (http://www.botany.wisc.edu/herbarium/) Four major floristic elements in the Wisconsin flora Boreal Alleghenian Ozarkian Prairie Two floristic provinces Northern hardwood Prairie forests Tension Zone Brief look at four plant communities Beech maple or southern mesic Oak forest or southern xeric Prairie Bog or fen Vascular Flora of Wisconsin 22 January 2009 Nomenclature and Vascular Cryptogams I Nomenclature vs. Classification Rank -

Intraspecific and Interspecific Genetic and Phylogenetic Relationships In
Theor Appl Genet (2005) 111: 1440–1456 DOI 10.1007/s00122-005-0076-2 ORIGINAL PAPER M. T. Cervera Æ V. Storme Æ A. Soto Æ B. Ivens M. Van Montagu Æ O. P. Rajora Æ W. Boerjan Intraspecific and interspecific genetic and phylogenetic relationships in the genus Populus based on AFLP markers Received: 11 March 2004 / Accepted: 24 June 2005 / Published online: 7 October 2005 Ó Springer-Verlag 2005 Abstract Although Populus has become the model genus pattern consistent with their known evolutionary rela- for molecular genetics and genomics research on forest tionships. A close relationship between Populus deltoides trees, genetic and phylogenetic relationships within this of the Aigeiros section and species of the Tacamahaca genus have not yet been comprehensively studied at the section was observed and, with the exception of Populus molecular level. By using 151 AFLPÒ (AFLPÒ is a wilsonii, between the species of the Leucoides, Taca- registered trademark of Keygene) markers, 178 acces- mahaca, and Aigeiros sections. Populus nigra was clearly sions belonging to 25 poplar species and three inter- separated from its consectional P. deltoides, and should specific hybrids were analyzed, using three accessions be classified separately from P. deltoides. The AFLP belonging to two willow species as outgroups. The ge- profiles pointed out to the lack of divergence between netic and phylogenetic relationships were generally some species and revealed that some accessions corre- consistent with the known taxonomy, although notable sponded with interspecific hybrids. This molecular study exceptions were observed. A dendrogram as well as a provides useful information about genetic relationships single most parsimonious tree, ordered the Populus among several Populus species and, together with mor- sections from the oldest Leuce to the latest Aigeiros,a phological descriptions and crossability, it may help re- view and update systematic classification within the Populus genus.