Contributions of Physical and Biogeochemical Processes to Phytoplankton Biomass Enhancement in the Surface and Subsurface Layers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topographic Influence on the Motion of Tropical Cyclones Landfalling on the Coast of China
OCTOBER 2016 C H E N A N D W U 1615 Topographic Influence on the Motion of Tropical Cyclones Landfalling on the Coast of China XIAOYU CHEN Key Laboratory of Meteorological Disaster, Ministry of Education/Joint International Research Laboratory of Climate and Environment Change/Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters/Pacific Typhoon Research Center, Nanjing University of Information Science and Technology, Nanjing, China LIGUANG WU Key Laboratory of Meteorological Disaster, Ministry of Education/Joint International Research Laboratory of Climate and Environment Change/Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters/Pacific Typhoon Research Center, Nanjing University of Information Science and Technology, Nanjing, and State Key Laboratory of Severe Weather, Chinese Academy of Meteorological Sciences, Beijing, China (Manuscript received 17 March 2016, in final form 10 August 2016) ABSTRACT A few recent numerical studies investigated the influence of a continent, which is much larger in size than islands, on the track of a landfalling tropical cyclone. It has been found that land surface roughness and temperature tend to make a tropical cyclone move toward the continent. In this study, the effect of the elevated terrain on the western part of mainland China on landfall tracks is examined through idealized numerical experiments, in which an initially axisymmetric vortex is embedded into a monsoon trough and takes a landfall track across China’s mainland. It is found that the effect of the elevated terrain on the western part of mainland China can enhance the southerly environmental flows in the low and midtroposphere, leading to an additional landward component of tropical cyclone motion, suggesting that forecasts for tropical cyclone tracks over land should take into consideration the change in thermal condition over the continent. -

Risk Reduction and Management in Escalating Water Hazards: How Fare the Poor?
Risk Reduction and Management in Escalating Water Hazards: How Fare the Poor? Leonardo Q. Liongson, PhD The article aims to take stock of and rapidly assess the human and economic damages brought about, not only by Typhoon Yolanda, but also by the recent Bohol 7.2 magnitude earthquake and its aftershocks during the period October-November 2013, and comparatively, the most recent typhoons and monsoons (habagat) rainstorm and flood events in the 21st century. It will also cover the positive new steps and efforts of the infrastructure and S&T arms of the national government, and the needed additional steps and tasks which must follow, for alleviating and mitigating the hazard risks of water-based natural disasters, with emphasis on helping and protecting the most exposed and vulnerable to the hazard risks, being the poor sector of the society. The article has emphasized the need for implementing structural mitigation measures in poor unprotected towns and regions in the country, especially under the challenge and threat posed by growing population and climate change. Likewise, non-structural mitigation measures (which have shorter gestation periods of months and few years only, compared to decades for major structural measures) must be provided under the imperative or necessity implied by the structural gap of existing structures to adequately reduce and effectively manage the increasing flood and storm surge hazard risks, caused by growing population and climate change. NO PRIOR WARNING OF SUPER STORM SURGES of 225 kilometers per hour (kph) and gusts of 260 kph coming from PAGASA, with the attendant rains and the Many days before the first landfall of super Typhoon wind-blown piled-up sea waves hitting the coastal areas Haiyan (Yolanda) in Samar and Leyte (in Region 8) last of the region. -

Genesis of Twin Tropical Cyclones As Revealed by a Global Mesoscale
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 117, D13114, doi:10.1029/2012JD017450, 2012 Genesis of twin tropical cyclones as revealed by a global mesoscale model: The role of mixed Rossby gravity waves Bo-Wen Shen,1,2 Wei-Kuo Tao,2 Yuh-Lang Lin,3 and Arlene Laing4 Received 12 January 2012; revised 26 April 2012; accepted 29 May 2012; published 12 July 2012. [1] In this study, it is proposed that twin tropical cyclones (TCs), Kesiny and 01A, in May 2002 formed in association with the scale interactions of three gyres that appeared as a convectively coupled mixed Rossby gravity (ccMRG) wave during an active phase of the Madden-Julian Oscillation (MJO). This is shown by analyzing observational data, including NCEP reanalysis data and METEOSAT 7 IR satellite imagery, and performing numerical simulations using a global mesoscale model. A 10-day control run is initialized at 0000 UTC 1 May 2002 with grid-scale condensation but no sub-grid cumulus parameterizations. The ccMRG wave was identified as encompassing two developing and one non-developing gyres, the first two of which intensified and evolved into the twin TCs. The control run is able to reproduce the evolution of the ccMRG wave and thus the formation of the twin TCs about two and five days in advance as well as their subsequent intensity evolution and movement within an 8–10 day period. Five additional 10-day sensitivity experiments with different model configurations are conducted to help understand the interaction of the three gyres, leading to the formation of the TCs. These experiments -

February 2020 Ajet
AJET News & Events, Arts & Culture, Lifestyle, Community FEBRUARY 2020 Riding the Jiu-Jitsu Wave Working for the Kyoryokutai The Changing Colors of the Red and White Singing Battle Journey Through Magic Embarrassing Adventures of an Expat in Tokyo The Japanese Lifestyle & Culture Magazine Written by the International Community in Japan1 In response to ongoing global news, the team at Connect Magazine would like to acknowledge the devastating impact of the 2019-2020 bushfires in Australia. Our thoughts and support are with those suffering. 2 Since September 2019, the raging fires across the eastern and southeastern Australian coastal regions have burned over 17.9 million acres, destroyed over 2000 homes, and killed least 27 people. A billion animals have been caught in the fires, with some species now pushed to the brink of extinction. Skies are reddened from air heavy with smoke— smoke which can be seen 2,000km away in New Zealand and even from Chile, South America, which is more than 11,000km away. Currently, massive efforts are being taken to tackle the bushfires and protect people, animals, and homes in the vicinity. If you would like to be a part of this effort, here are some resources you can use to help: Country Fire Authority Country Fire Service Foundation In Victoria In South Australia New South Wales Rural The Australian Red Cross Fire Service Fire recovery and relief fund World Wildlife Fund GIVIT Caring for injured wildlife and Donating items requested by habitat restoration those affected The Animal Rescue Collective Craft Guild Making bedding and bandaging for injured animals. -

4. the TROPICS—HJ Diamond and CJ Schreck, Eds
4. THE TROPICS—H. J. Diamond and C. J. Schreck, Eds. Pacific, South Indian, and Australian basins were a. Overview—H. J. Diamond and C. J. Schreck all particularly quiet, each having about half their The Tropics in 2017 were dominated by neutral median ACE. El Niño–Southern Oscillation (ENSO) condi- Three tropical cyclones (TCs) reached the Saffir– tions during most of the year, with the onset of Simpson scale category 5 intensity level—two in the La Niña conditions occurring during boreal autumn. North Atlantic and one in the western North Pacific Although the year began ENSO-neutral, it initially basins. This number was less than half of the eight featured cooler-than-average sea surface tempera- category 5 storms recorded in 2015 (Diamond and tures (SSTs) in the central and east-central equatorial Schreck 2016), and was one fewer than the four re- Pacific, along with lingering La Niña impacts in the corded in 2016 (Diamond and Schreck 2017). atmospheric circulation. These conditions followed The editors of this chapter would like to insert two the abrupt end of a weak and short-lived La Niña personal notes recognizing the passing of two giants during 2016, which lasted from the July–September in the field of tropical meteorology. season until late December. Charles J. Neumann passed away on 14 November Equatorial Pacific SST anomalies warmed con- 2017, at the age of 92. Upon graduation from MIT siderably during the first several months of 2017 in 1946, Charlie volunteered as a weather officer in and by late boreal spring and early summer, the the Navy’s first airborne typhoon reconnaissance anomalies were just shy of reaching El Niño thresh- unit in the Pacific. -

6 2. Annual Summaries of the Climate System in 2009 2.1 Climate In
2. Annual summaries of the climate system in above normal in Okinawa/Amami because hot and 2009 sunny weather was dominant under the subtropical high in July and August. 2.1 Climate in Japan (d) Autumn (September – November 2009, Fig. 2.1.1 Average surface temperature, precipitation 2.1.4d) amounts and sunshine durations Seasonal mean temperatures were near normal in The annual anomaly of the average surface northern and Eastern Japan, although temperatures temperature over Japan (averaged over 17 observatories swung widely. In Okinawa/Amami, seasonal mean confirmed as being relatively unaffected by temperatures were significantly above normal due to urbanization) in 2009 was 0.56°C above normal (based the hot weather in the first half of autumn. Monthly on the 1971 – 2000 average), and was the seventh precipitation amounts were significantly below normal highest since 1898. On a longer time scale, average nationwide in September due to dominant anticyclones. surface temperatures have been rising at a rate of about In contrast, in November, they were significantly 1.13°C per century since 1898 (Fig. 2.1.1). above normal in Western Japan under the influence of the frequent passage of cyclones and fronts around 2.1.2 Seasonal features Japan. In October, Typhoon Melor (0918) made (a) Winter (December 2008 – February 2009, Fig. landfall on mainland Japan, bringing heavy rainfall and 2.1.4a) strong winds. Since the winter monsoon was much weaker than (e) December 2009 usual, seasonal mean temperatures were above normal In the first half of December, temperatures were nationwide. In particular, they were significantly high above normal nationwide, and heavy precipitation was in Northern Japan, Eastern Japan and Okinawa/Amami. -

Tropical Cyclone Intensity Estimation Using Multi-Dimensional Convolutional Neural Networks from Geostationary Satellite Data
remote sensing Article Tropical Cyclone Intensity Estimation Using Multi-Dimensional Convolutional Neural Networks from Geostationary Satellite Data Juhyun Lee 1, Jungho Im 1,* , Dong-Hyun Cha 1, Haemi Park 2 and Seongmun Sim 1 1 School of Urban & Environmental Engineering in Ulsan National Institute of Science and Technology, Ulsan 44919, Korea; [email protected] (J.L.); [email protected] (D.-H.C.); [email protected] (S.S.) 2 Institute of Industrial Science in the University of Tokyo, A building, 4 Chome-6-1 Komaba, Meguro City, Tokyo 153-8505, Japan; [email protected] * Correspondence: [email protected]; Tel.: +82-52-217-2824 Received: 25 November 2019; Accepted: 25 December 2019; Published: 28 December 2019 Abstract: For a long time, researchers have tried to find a way to analyze tropical cyclone (TC) intensity in real-time. Since there is no standardized method for estimating TC intensity and the most widely used method is a manual algorithm using satellite-based cloud images, there is a bias that varies depending on the TC center and shape. In this study, we adopted convolutional neural networks (CNNs) which are part of a state-of-art approach that analyzes image patterns to estimate TC intensity by mimicking human cloud pattern recognition. Both two dimensional-CNN (2D-CNN) and three-dimensional-CNN (3D-CNN) were used to analyze the relationship between multi-spectral geostationary satellite images and TC intensity. Our best-optimized model produced a root mean squared error (RMSE) of 8.32 kts, resulting in better performance (~35%) than the existing model using the CNN-based approach with a single channel image. -
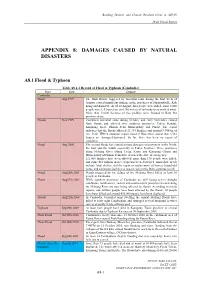
Appendix 8: Damages Caused by Natural Disasters
Building Disaster and Climate Resilient Cities in ASEAN Draft Finnal Report APPENDIX 8: DAMAGES CAUSED BY NATURAL DISASTERS A8.1 Flood & Typhoon Table A8.1.1 Record of Flood & Typhoon (Cambodia) Place Date Damage Cambodia Flood Aug 1999 The flash floods, triggered by torrential rains during the first week of August, caused significant damage in the provinces of Sihanoukville, Koh Kong and Kam Pot. As of 10 August, four people were killed, some 8,000 people were left homeless, and 200 meters of railroads were washed away. More than 12,000 hectares of rice paddies were flooded in Kam Pot province alone. Floods Nov 1999 Continued torrential rains during October and early November caused flash floods and affected five southern provinces: Takeo, Kandal, Kampong Speu, Phnom Penh Municipality and Pursat. The report indicates that the floods affected 21,334 families and around 9,900 ha of rice field. IFRC's situation report dated 9 November stated that 3,561 houses are damaged/destroyed. So far, there has been no report of casualties. Flood Aug 2000 The second floods has caused serious damages on provinces in the North, the East and the South, especially in Takeo Province. Three provinces along Mekong River (Stung Treng, Kratie and Kompong Cham) and Municipality of Phnom Penh have declared the state of emergency. 121,000 families have been affected, more than 170 people were killed, and some $10 million in rice crops has been destroyed. Immediate needs include food, shelter, and the repair or replacement of homes, household items, and sanitation facilities as water levels in the Delta continue to fall. -

Japan's Insurance Market 2020
Japan’s Insurance Market 2020 Japan’s Insurance Market 2020 Contents Page To Our Clients Masaaki Matsunaga President and Chief Executive The Toa Reinsurance Company, Limited 1 1. The Risks of Increasingly Severe Typhoons How Can We Effectively Handle Typhoons? Hironori Fudeyasu, Ph.D. Professor Faculty of Education, Yokohama National University 2 2. Modeling the Insights from the 2018 and 2019 Climatological Perils in Japan Margaret Joseph Model Product Manager, RMS 14 3. Life Insurance Underwriting Trends in Japan Naoyuki Tsukada, FALU, FUWJ Chief Underwriter, Manager, Underwriting Team, Life Underwriting & Planning Department The Toa Reinsurance Company, Limited 20 4. Trends in Japan’s Non-Life Insurance Industry Underwriting & Planning Department The Toa Reinsurance Company, Limited 25 5. Trends in Japan's Life Insurance Industry Life Underwriting & Planning Department The Toa Reinsurance Company, Limited 32 Company Overview 37 Supplemental Data: Results of Japanese Major Non-Life Insurance Companies for Fiscal 2019, Ended March 31, 2020 (Non-Consolidated Basis) 40 ©2020 The Toa Reinsurance Company, Limited. All rights reserved. The contents may be reproduced only with the written permission of The Toa Reinsurance Company, Limited. To Our Clients It gives me great pleasure to have the opportunity to welcome you to our brochure, ‘Japan’s Insurance Market 2020.’ It is encouraging to know that over the years our brochures have been well received even beyond our own industry’s boundaries as a source of useful, up-to-date information about Japan’s insurance market, as well as contributing to a wider interest in and understanding of our domestic market. During fiscal 2019, the year ended March 31, 2020, despite a moderate recovery trend in the first half, uncertainties concerning the world economy surged toward the end of the fiscal year, affected by the spread of COVID-19. -

An Introduction to Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) Organizations in Taiwan
CENTER FOR EXCELLENCE IN DISASTER MANAGEMENT & HUMANITARIAN ASSISTANCE An Introduction to Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) Organizations in Taiwan WWW.CFE-DMHA.ORG Contents Introduction ...........................................................................................................................2 Humanitarian Assistance and Disaster Relief (HADR) Organizations ..................................3 Search and Rescue (SAR) Organizations ..........................................................................18 Appendix A: Taiwan Foreign Disaster Relief Assistance ....................................................29 Appendix B: DOD/USINDOPACOM Disaster Relief in Taiwan ...........................................31 Appendix C: Taiwan Central Government Disaster Management Structure .......................34 An Introduction to Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) Organizations in Taiwan 1 Introduction This information paper serves as an introduction to the major Humanitarian Assistance and Disaster Relief (HADR) and Search and Rescue (SAR) organizations in Taiwan and international organizations working with Taiwanese government organizations or non-governmental organizations (NGOs) in HADR. The paper is divided into two parts: The first section focuses on major International Non-Governmental Organizations (INGOs), and local NGO partners, as well as international Civil Society Organizations (CSOs) working in HADR in Taiwan or having provided -

Paleo-Tropical Cyclone Deposits in the Taiwan Strait
Paleo‐tropical cyclone deposits in the Taiwan Strait: Processes, examples, and conceptual model Shahin E. Dashtgard, Ludvig Löwemark, Romain Vaucher, Yuyen Pan, Jessica Pilarcyzk, and Sebastien Castelltort Supplementary Data File Part A: Summary of all tropical cyclones that impacted Taiwan between 1982 and 2017 Summary of Typhoons in Taiwan 1982 to 2017 ** The number of events refers to how many times each storm was counted. For example, if a storm cross Taiwan through the central area and then the northern area (Fig. 2) it would be counted twice. Storms for which the eye was more than 50 km from the shoreline are only counted once based on the closest point of the eye to the shoreline. If the closest point is in the south, then the event is assigned to the south region (Fig. 7). OL = overland CE = central‐east TD = tropical Depression N = north CW = central‐west TS = Tropical Storm NE = northeast S = south TY = typhoon NW = northwest SE = southeast STY = super‐typhoon Acronyms C = central SW = southwest No. Typhoon Name Start Date Strongest Level of Typhoon Closest Distance Posistion Relative Number of level of at closest point / to eye to Taiwan to Taiwan events by Year Typhoon landfall Margin (km) area 1TS 06W 1982 ‐ TESS 1982‐06‐28 TS TD 158 SW 1 2TS 08W 1982 ‐ VAL 1982‐07‐04 TS TS 154 NE 1 3TY 10W 1982 ‐ ANDY 1982‐07‐21 TY4 TY2 0 OL ‐ S1 4TY 12W 1982 ‐ CECIL 1982‐08‐04 TY4 TY3 152 NE 1 5TY 13W 1982 ‐ DOT 1982‐08‐08 TY1 TS 0 OL ‐ S1 6TY 15W 1982 ‐ FAYE 1982‐08‐20 TY2 TD 39 S 1 7 1983 STY 04W 1983 ‐ WAYNE 1983‐07‐20 STY4/5 TY2/3 80 -

Radar Observation of Precipitation Asymmetries in Tropical Cyclones Making Landfall on East China Coast
MAY 2013 WU ET AL. 81 RADAR OBSERVATION OF PRECIPITATION ASYMMETRIES IN TROPICAL CYCLONES MAKING LANDFALL ON EAST CHINA COAST 1,2 2,* 3 4 DAN WU , KUN ZHAO , BEN JONG -DAO JOU , WEN -CHAU LEE 1Shanghai Typhoon Institute/China Meteorological Administration, Shanghai 2Key Laboratory for Mesoscale Severe Weather/MOE and School of Atmospheric Science, Nanjing University, Nanjing 3Department of Atmospheric Sciences, National Taiwan University, Taipei 4The National Center for Atmospheric Research, Boulder ABSTRACT This study explores, for the first time, the asymmetric distribution of precipitation in tropical cyclones (TCs) making landfall along east China coast using reflectivity data collected from coastal Doppler radars at mainland China and Taiwan. Six TCs (Saomai, Khanun, Wipha, Matsa, Rananim and Krosa) from 2004 to 2007 are ex- amined. The temporal and spatial evolution of these TCs’ inner and outer core asymmetric precipitation patterns before and after landfall is investigated. The radius of inner-core region is a function of the size of a TC apart from a fixed radius (100 km) adopted in previous studies. All six TCs possessed distinct asymmetric precipitation patterns between the inner- and outer- core regions. The amplitude of asymmetry decreases with the increasing TC intensity and it displays an ascending (descend- ing) trend in the inner (outer) core. In the inner-core region, the heavy rainfall with reflectivity factor above 40 dBZ tends to locate at the downshear side before landfall. Four cases have precipitation maxima on the downshear left side, in agreement with previous studies. As TCs approaching land (~ 2 hr before landfall), their precipitation maxima generally shift to the front quadrant of the motion partly due to the interaction of TC with the land surface.