Chemistry Dictionary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dealloyed Pt Core-Shell Nanoparticles
The Strem Product Line OUR LINE OF RESEARCH CHEMICALS Custom Synthesis Biocatalysts & Organocatalysts Electronic Grade Chemicals cGMP Facilities Fullerenes High Purity Inorganics & Alkali Metals FDA Inspected Ionic Liquids Ligands & Chiral Ligands Drug Master Files Metal Acetates & Carbonates Metal Alkoxides & beta-Diketonates Complete Documentation Metal Alkyls & Alkylamides Metal Carbonyls & Derivatives Metal Catalysts & Chiral Catalysts Metal Foils, Wires, Powders & Elements Metal Halides, Hydrides & Deuterides Metal Oxides, Nitrates, Chalcogenides Metal Scavengers Metallocenes Nanomaterials Organofluorines Organometallics Organophosphines & Arsines Porphines & Phthalocyanines Precious Metal & Rare Earth Chemicals Volatile Precursors for MOCVD, CVD & ALD Strem Chemicals, Inc. Strem Chemicals, Inc. 7 Mulliken Way 15, rue de l’Atome Dexter Industrial Park Zone Industrielle Newburyport, MA 01950-4098 F-67800 BISCHHEIM (France) U.S.A. Tel.: +33 (0) 3 88 62 52 60 Fax: +33 (0) 3 88 62 26 81 Office Tel: (978) 499-1600 Email: [email protected] Office Fax: (978) 465-3104 Toll-free (U.S. & Canada) Strem Chemicals, Inc. Tel: (800) 647-8736 Postfach 1215 Fax: (800) 517-8736 D-77672 KEHL, Germany Tel.: +49 (0) 7851 75879 Dealloyed Pt core-shell nanoparticles: Email: [email protected] Fax: +33 (0) 3 88 62 26 81 Active and durable electrocatalysts for low-temperature Email: [email protected] www.strem.com Polymer Electrolyte Membrane Fuel Cells (PEMFCs) Strem Chemicals UK, Ltd. by Professor Dr. Peter Strasser An Independent Distributor of Strem Chemicals Products Newton Hall, Town Street Strem Chemicals Chemist Finds Newton, Cambridge, CB22 7ZE, UK Tel.: +44 (0)1223 873 028 New Way to Drive to Work Fax: +44 (0)1223 870 207 by Dr. -
![(12) United States Patent (10) Patent N0.: US 8,304,580 B2 Nanmyo Et A]](https://docslib.b-cdn.net/cover/4589/12-united-states-patent-10-patent-n0-us-8-304-580-b2-nanmyo-et-a-144589.webp)
(12) United States Patent (10) Patent N0.: US 8,304,580 B2 Nanmyo Et A]
US008304580B2 (12) United States Patent (10) Patent N0.: US 8,304,580 B2 Nanmyo et a]. (45) Date of Patent: Nov. 6, 2012 (54) METHOD FOR PRODUCING TRIS(PER FOREIGN PATENT DOCUMENTS FLUORO-ALKANESULFONYL)METHIDE EP 0813521 B1 * 9/2000 ACID SALT JP 2000-226392 A 8/2000 JP 2000-256348 A 9/2000 (75) Inventors: Tsutomu Nanmyo, Ube (JP); Shintaro JP 2000-256348 A * 9/2000 Sasaki, Ube (JP); Takashi Kume, OTHER PUBLICATIONS KaWagoe (JP) English translation of JP-2000-256348-A; “machine translation” (73) Assignee: Central Glass Company, Limited, from JPO link at: http://WWW4.ipd1.inpit.go.jp/Tokujitu/ Ube-shi (JP) tj sogodbenkipdl accessed Oct. 25, 201 1; relevant part of document.* European Search Report dated Jun. 8, 2011 (four (4) pages). ( * ) Notice: Subject to any disclaimer, the term of this Waller et al., “Tris (tri?uoromethanesulfonyl) methide (“Tri?ide”) patent is extended or adjusted under 35 Anion: Convenient Preparation, X-ray Crystal Structures, and U.S.C. 154(b) by 528 days. Exceptional Catalytic Activity as a Counterion With Ytterbium (III) and Scandium (111)”, Journal of Organic Chemistry, vol. 64, 1999, pp. (21) Appl. N0.: 12/520,17s 2910-2913, XP-002636992. Turowsky et al., “Tris ((tri?uoromethyl) sulfonyl) methane, HC (22) PCT Filed: Dec. 18, 2007 (SO2CF3)3”, Journal of Inorganic Chemistry, vol. 27, 1988, pp. 2135-2137, XP-002636993. (86) PCT No.: PCT/JP2007/074296 International Search Report and PCT/ISN237 W/translation dated Feb. 12, 2008 (Seven (7) pages). § 371 (0X1)’ LutZ Turowsky et al., “Tris((tri?uoromethyl)sulfonyl)methane, (2), (4) Date: Jun. -

Publikationsliste - List of Publications
Publikationsliste - List of Publications Philipp Gütlich (1) Die Reaktion von Aluminiumchlorid mit Chlorwasserstoff in 1,1,2,2- Tetrachloräthan und in Nitromethan Lieser, K.H.; Gütlich, P. Ber. Bunsenges. Phys. Chem. 1963, 67, 445 (2) Der Einfluß der Vorbehandlung auf den heterogenen Isotopenaustausch an derOberfläche von Ionenkristallen und die Bildung von Kationenkörpern und Anionenkörpern Gütlich, P.; Lieser, K.H. Z. Phys. Chem. (Neue Folge) 1965, 46, 3/4, 216 (3) Die spezifische Oberfläche von frisch gefälltem Bariumsulfat Gütlich, P; Lieser, K.H. Z. Phys. Chem. (Neue Folge) 1965, 46, 5/6, 257 (4) Die Geschwindigkeit des heterogenen Isotopenaustausches an der Oberfläche von Ionenkristallen Lieser, K.H.; Gütlich, P.; Rosenbaum, I. Radiochim. Acta 1965, 4, 216 (5) Die Temperaturabhängigkeit des heterogenen Isotopenaustausches an der Oberfläche von Ionenkristallen Lieser, K.H.; Gütlich, P.; Rosenbaum, I. Radiochim. Acta 1966, 5, 38 (6) Kinetics and Mechanism of Heterogeneous Exchange Reactions on the Surface of Ionic Crystals K.H. Lieser, K.H.; Gütlich, P.; Rosenbaum, I. Proc. Int. Atomic Energy Agency, Symp. "Exchange Reactions", Brookhaven 1965 (7) Exchange Equilibria on the Surface of Ionic Crystals Lieser, K.H.; Gütlich, P.; Hild, W.; Hecker, A.; Rosenbaum, I. Proc. Int. Atomic Energy Agency, Symp. "Exchange Reactions", Brookhaven 1965 (8) Hot-Atom Reaction Products in Crystals of Hexa- and Trivalent Chromium Compounds Gütlich, P.; Harbottle, G. Radiochim. Acta 1966, 5, 70 51 (9) A Study of Cr Retention and Annealing in Single Crystals of K2CrO4 Irradiated at Low Neutron Doses Gütlich, P.; Harbottle, G. Radiochim. Acta 1967, 8, 30 (10) Beiträge zur Anwendung des Mößbauer-Effekts in der Chemie Gütlich, P. -

(12) United States Patent (10) Patent No.: US 8,333,879 B2 M00re Et Al
US0083.33879B2 (12) United States Patent (10) Patent No.: US 8,333,879 B2 M00re et al. (45) Date of Patent: Dec. 18, 2012 (54) ELECTRODEPOSITION OF DELECTRIC (56) References Cited COATINGS ON SEMCONDUCTIVE SUBSTRATES U.S. PATENT DOCUMENTS 3,455,806 A 7/1969 Spoor et al. (75) Inventors: Kelly L. Moore, Dunbar, PA (US); 3,663,389 A 5, 1972 Koral et al. Michael J. Pawlik, Glenshaw, PA (US); 3,749,657 A 7, 1973 Le Bras et al. Michael G. Sandala, Pittsburgh, PA 3,793,278 A 2f1974 De Bona (US); Craig A. Wilson, Allison Park, PA 3,928, 157 A 12/1975 Suematsu et al. 3,947.338 A 3, 1976 Jerabek et al. (US) 3,947,339 A 3, 1976 Jerabek et al. 3,962,165 A 6, 1976 Bosso et al. (73) Assignee: PPG Industries Ohio, Inc., Cleveland, 3,975,346 A 8, 1976 Bosso et al. OH (US) 3,984,299 A 10, 1976 Jerabek (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. EP OO12463 A1 6, 1980 (21) Appl. No.: 13/240,455 OTHER PUBLICATIONS (22) Filed: Sep. 22, 2011 Kohler, E. P. "An Apparatus for Determining Both the Quantity of Gas Evolved and the Amount of Reagent Consumed in Reactions (65) Prior Publication Data with Methyl Magnesium Iodide'. J. Am. Chem. Soc., 1927, 49 (12), US 2012/OOO6683 A1 Jan. 12, 2012 3181-3188, American Chemical Society, Washington, D.C. Related U.S. -

Safety Data Sheet
SAFETY DATA SHEET Revision Date 29-Jun-2018 Revision Number 1 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identification Product Description: Cadmium arsenide Cat No. : 22773 CAS-No 12006-15-4 Molecular Formula As2 Cd3 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Alfa Aesar . Avocado Research Chemicals, Ltd. Shore Road Port of Heysham Industrial Park Heysham, Lancashire LA3 2XY United Kingdom Office Tel: +44 (0) 1524 850506 Office Fax: +44 (0) 1524 850608 E-mail address [email protected] www.alfa.com Product Safety Department 1.4. Emergency telephone number Call Carechem 24 at +44 (0) 1865 407333 (English only); +44 (0) 1235 239670 (Multi-language) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Based on available data, the classification criteria are not met Health hazards Acute oral toxicity Category 3 (H301) Acute Inhalation Toxicity - Dusts and Mists Category 2 (H330) Environmental hazards ______________________________________________________________________________________________ ALFAA22773 Page 1 / 10 SAFETY DATA SHEET Cadmium arsenide Revision Date 29-Jun-2018 ______________________________________________________________________________________________ Acute aquatic toxicity Category 1 (H400) Chronic -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -
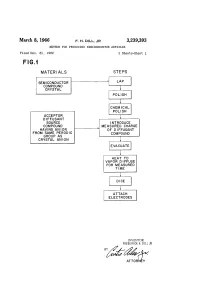
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD for PRODUCING SEMICONDUCTOR ARTICLES Filed Dec
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD FOR PRODUCING SEMICONDUCTOR ARTICLES Filed Dec. 31, 1962 2. Sheets-Sheet FG. MATER ALS STEPS SEMCONDUCTOR COMPOUND CRYSTAL POLISH CHEMICAL POLISH ACCEPTOR DFFUSANT SOURCE INTRODUCE COMPOUND MEASURED CHARGE HAVING AN ON OF DFFUSANT FROM SAME PERODC COMPOUND GROUP AS CRYSTAL ANION EVACUATE HEAT TO VAPOR DIFFUSE FOR MEASURED TIME ATTACH ELECTRODES INVENTOR. FREDERICK H. DLL JR ATTORNEY March 8, 1966 F., H., DL, JR 3,239,393 METHOD FOR PRODUCING SEMCONDUCTOR ARTICLES Filed Dec. 31, 962 2. Sheets-Sheet 2 Šes is series NNNNN r N NNNNNNNN 3,239,393 United States Patent Office Patented Mar. 8, 1966 2. to produce precisely the desired results. For instance, 3,239,393 when metallic zinc is used as the diffusant material for METHOD FOR PRODUCING SEMCONDUCTOR a gallium arsenide substrate, it is very difficult to obtain ARTICLES the pure zinc metal with no zinc oxide film upon the metal. Frederick H. Dii, Jr., Patnam Waley, N.Y., assigor to 5 Furthermore, the metal is so tough that it is difficult to International Business Machines Corporation, New divide a pure metal sample into smaller pieces in order York, N.Y., a corporation of New York to obtain exactly the correct quantity for the diffusion Filed Dec. 31, 1962, Ser. No. 248,679 process. The zinc oxide on the surface of the metallic Zinc 7 Claims. (C. 48-189) diffusant material is very undesirable for a number of This invention relates to an improved diffusion process IO reasons. The oxygen is not wanted in the diffusion Vapor, for the production of Semiconductor devices, and more and the zinc oxide tends to form a protective coating particularly to an improved vapor diffusion process in over the zinc which inhibits the formation of the desired which the introduction of unwanted impurities is very zinc metal vapor which is required for the diffusion proc effectively and simply avoided, and which possesses other CSS. -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

Crystal Structure Transformations in Binary Halides
1 A UNITED STATES DEPARTMENT OF A111D3 074^50 IMMERCE JBLICAT10N NSRDS—NBS 41 HT°r /V\t Co^ NSRDS r #C£ DM* ' Crystal Structure Transformations in Binary Halides u.s. ARTMENT OF COMMERCE National Bureau of -QC*-| 100 US73 ho . 4 1^ 72. NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation’s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation’s physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—Linac Radiation 2—Nuclear Radiation 2—Applied Radiation 2—Quantum Electronics 3— Electromagnetics 3—Time and Frequency 3—Laboratory Astrophysics 3—Cryo- 3 genics . -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Thermomagnetic Effects in Cadmium Arsenide
Thermomagnetic effects in cadmium arsenide Citation for published version (APA): Blom, F. A. P. (1970). Thermomagnetic effects in cadmium arsenide. Technische Hogeschool Eindhoven. https://doi.org/10.6100/IR155506 DOI: 10.6100/IR155506 Document status and date: Published: 01/01/1970 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -
![Synthesis, Separation, Structural and DFT Studies of [Re6- Xmoxse8(CN)6]N](https://docslib.b-cdn.net/cover/1606/synthesis-separation-structural-and-dft-studies-of-re6-xmoxse8-cn-6-n-2201606.webp)
Synthesis, Separation, Structural and DFT Studies of [Re6- Xmoxse8(CN)6]N
CO-TUTELLE THESE DE DOCTORAT DE L'UNIVERSITE DE RENNES 1 OMUE NIVERSITE RETAGNE OIRE C U B L ECOLE DOCTORALE N° 596 Matière Molécules et Matériaux Spécialité : Chimie Inorganique NIKOLAEV INSTITUTE OF INORGANIC CHEMISTRY (NIIC) Novosibirsk, Russia Par Viktoria Muravieva Briques moléculaires à clusters hétérométalliques chalcogénées {Re 6-xMoxSe8} x = 1-3 : cristallochimie, structures electroniques et propriétés redox Thèse présentée et soutenue à Novosibirsk, le 27 novembre 2019 Unité de recherche : Institut des Sciences Chimiques de Rennes – UMR CNRS 6226 Nikolaev Institute of Inorganic Chemistry, Novosibirsk Rapporteurs avant soutenance : Composition du Jury : Emmanuel CADOT Emmanuel CADOT Professeur, Université de Versailles Professeur, Université de Versailles / rapporteur Sylvie FERLAY Vladimir FEDIN Professeur, Université de Strasbourg Professeur, NIIC de Novosibirsk / examinateur Stéphane CORDIER Directeur de recherches CNRS, Université de Rennes 1 / Directeur de thèse Nikolay NAUMOV Professeur, NIIC de Novosibirsk/ Co-directeur de thèse Maxim SOKOLOV Professeur, Novosibirsk State University / examinateur Galina ROMANENKO Researcher, International Tomography Center, Novosibirsk / examinatrice Membres invités: Pierric LEMOINE Chargé de Recherches CNRS, Université de Rennes 1 1 Carmelo PRESTIPINO Chargé de Recherche CNRS, Université de Rennes 1 1 Contents Résumé détaillé de la thèse ................................................................................................ 5 The list of Acronyms .......................................................................................................